
Risperidone Oral Solution
Key words:
Tablets, capsules, granules, topical solutions, oral solutions, suppositories, ointments
Classification:
Product Description
Instructions for Risperidone Oral Solution
Warning
Increasing mortality in elderly patients with dementia-related psychosis The risk of death is increased when elderly patients with dementia-related psychosis are treated with atypical antipsychotics compared with placebo. Studies have shown that, similar to atypical antipsychotics, treatment with typical antipsychotics may increase mortality. It is not clear whether the increase in mortality in the studies is due to antipsychotic drugs or some characteristic of the patients themselves. Risperidone is not approved for the treatment of patients with dementia-related psychosis (see Precautions)
[Name of Drug]]
Generic Name: Risperidone Oral Solution
英文名称:Risperidone Oral Solution
汉语拼音:Lipeitong Koufurongye
[ingredients]]
Chemical name: 3-[2-[4-(6-1,2-benzisoxazol -3-yl)-1-piperidinyl] ethyl]-6.7,8,9-tetrahydro -2-methyl -4H-pyridyl [1,2-0] pyrimidin-4-one
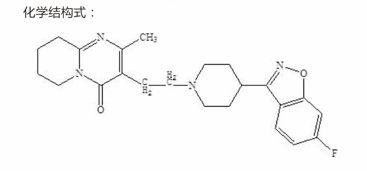
Formula: C H F N.O
Molecular weight: 410.49
Excipients: tartaric acid, benzoic acid, sodium hydroxide and purified water.
character]
This product is a colorless clear liquid.
indications]
Adults and 13 to 17 years old adolescents with schizophrenia, for the acute phase of effective treatment of patients, in the maintenance phase of treatment, this product can continue to play its clinical efficacy;
Manic episodes of bipolar disorder in adults and children and adolescents aged 10 to 17 years, as monotherapy or in combination with lithium or valproate; autism-related irritability in children and adolescents aged 5 to 17 years;
Persistent aggression or other destructive behavior associated with mental retardation or mental retardation and conduct disorder in children and adolescents aged 5 to 17 years.
[specification]]
0.1%(30ml:30mg)
Usage and dosage]
Adults and Adolescents Aging 13-17 Schizophrenia
Adults:
1 or 2 times daily.
The starting dose of recommend is 2 times a day, 1mg each time, and the second day is increased to 2 times a day, 2mg each time; If it can be tolerated, it can be increased to 3mg twice a day on the third day. After that, the dose can be maintained unchanged or further adjusted according to the patient's condition.
The recommend dose of risperidone is 4 to 8m9 per day. The effective dose range of risperidone is 4~16mg per day, but it should be noted that the dose exceeding 6mg per day (according to the regimen of taking twice a day) may not achieve better curative effect than the lower dose, and more extrapyramidal symptoms or other adverse reactions may occur. Therefore, the dose should be reasonably selected according to the patient's response. If there is no definite necessity, the dose exceeding 6mg per day is generally not recommend. The safety of doses above 16mg daily has not been evaluated; therefore, risperidone should not be dosed above 16mg daily.
The results of clinical trials show that the effective dose of risperidone to delay the recurrence of schizophrenia is 2-8mg per day, and the safety and effectiveness of once daily administration. In the trial, the initial dose was 1mg on the first day, increased to 2mg daily on the second day, and increased to 4mg daily on the third day. Thereafter, this dose may be maintained or further adjusted according to patient response.
In general, the dose adjustment of risperidone should be slow. The interval between dose adjustments is generally at least one week. When adjusting, the recommend single dose adjustment range is 1~2mg.
During treatment, the need for continued use and the appropriate dose should be evaluated periodically. When switching from other antipsychotics to this product, the original antipsychotic should be discontinued. The need for continued use of antiparkinsonian drugs should be reassessed periodically.
Adolescents (13-17 years):
The recommend starting dose is 0.5mg daily, given in a single dose in the morning or evening. If tolerated, the dose may be escalated in daily increments of 0,5mg or 1mg after intervals of 24 hours or more. The therapeutic dose of recommend is 3mg daily. Although the efficacy of doses of 1 to 6mg daily has been demonstrated in studies of adolescents with schizophrenia, no additional benefit has been seen at doses above 3mg daily, and higher doses are associated with more adverse events. Doses higher than 6mg daily have not been studied, and the need for continued use of this product and the appropriate dose should be evaluated periodically during treatment.
Episodes of bipolar disorder in adults and children and adolescents aged 10 to 17 years
Adults: once daily. The starting dose of recommend is 1~2mg once a day, and the ideal therapeutic dose for most patients is 2~6mg per day, which can be adjusted according to the needs of patients. The amplitude of the dose adjustment is 1mg per day, and the dose adjustment is at least 24 hours or more.
During treatment, the need for continued use and the appropriate dose should be evaluated periodically.
Children and adolescents (10-17 years):
The recommend starting dose is 0.5mg daily, given in a single dose in the morning or evening. If tolerated, the dose can be increased by 0.5mg or lmg per day after an interval of 24 hours or more, and the recommend therapeutic dose is 1-2.5mg per day. Although the efficacy of 0.5-6mg per day has been proved in the study of pediatric patients with bipolar disorder ankle mania, there is no additional benefit at doses above 2.5mg per day, and higher doses are associated with more adverse events. Doses higher than 6mg daily have not been studied.
During treatment, the need for continued use and the appropriate dose should be evaluated periodically.
Autism-related irritability in children and adolescents aged 5 to 17 years
1 or 2 times daily.
For patients weighing less than 20kg (more than 15kg), the initial dose of recommend is 0.25mg per day. After an interval of at least 4 days, the recommend dose can be increased to 0.5mg per day. After maintaining the above recommend dose for at least 14 days, if the patient does not obtain satisfactory clinical curative effect, the dose can be increased by 0.25mg per day after an interval of at least 2 weeks or more, up to 0.75mg per day, for patients with a body weight greater than or equal to 20kg, the recommend starting dose is 0.5mg per day. After at least an interval of 4 days, the recommend dose may be increased to 1mg daily. After maintaining the above recommend dose for at least 14 days, if the patient does not obtain satisfactory clinical efficacy, the dose can be increased by 0.5mg per day up to 1.5mg per day after an interval of at least two weeks or more. The effective dose range is 0.5mg ~ 3mg/day.
There are no study data on the dose administered to patients weighing less than 15kg.
During the treatment period, the necessity and appropriate dosage of this product should be evaluated regularly. When the recommend dose is reached and the clinical efficacy can be maintained, the dose can be gradually reduced to obtain the best balance between effectiveness and safety. Physicians should regularly assess the long-term risks and benefits of taking the drug.
Patients with persistent drowsiness are best to take risperidone before bedtime, once a day, or divide the daily dose into 2 doses, or reduce the dose as appropriate.
Persistent aggression or other destructive behavior associated with mental retardation or mental retardation and conduct disorder in children and adolescents aged 5 to 17 years
It is suitable for short-term treatment (up to 6 weeks) of children and adolescents over 5 years of age with persistent aggression, diagnosed as below-average intelligence or mental retardation according to DSM criteria, and the severity of aggressive or other disruptive behavior in these patients should be To the extent that medication is required. Medication should be an integral part of a more comprehensive treatment programme, including psychosocial and educational interventions. It is recommended that risperidone be prescribed by a child and adolescent neurologist or psychiatrist or a physician familiar with the treatment of behavioral disorders in children and adolescents. For patients weighing less than 50kg, the recommend starting dose is 0.25mg per day. If necessary, the dose can be escalated by 0.25mg per day, and the therapeutic dose range is 0.25mg to 0.75mg per day.
For patients with a body weight greater than or equal to 50kg, the recommend starting dose is 0.5mg per day. If necessary, the dose can be escalated by 0.5mg per day. The therapeutic dose ranges from 0.5mg to 1.5mg daily, with the optimal therapeutic dose being 1mg daily for most patients.
During treatment, the need for continued use and the appropriate dose should be evaluated periodically.
Use in patients with kidney and liver disease
The ability to clear the active ingredients of antipsychotics is lower in patients with kidney disease than in normal adults, while the concentration of the unbound fraction of risperidone in the plasma of patients with liver disease is higher than in normal subjects.
The initial dose and maintenance dose of patients with kidney disease and liver disease should be halved, and the dose adjustment range and speed should be reduced. It is recommended that the initial dose be 0.5mg twice a day. According to the needs of patients, the dose can be gradually increased to twice a day, 1~2mg each time. The dose adjustment interval should be at least one week. The range of dose increase and decrease is 0.5mg twice daily. The clinical application experience of patients with kidney disease and liver disease is limited, and medication should be used carefully.
adverse reaction]
1. The common adverse reactions related to taking this product are: insomnia, anxiety, agitation,
Headache, dry mouth, 2, less common adverse reactions are: drowsiness, fatigue, decreased attention, constipation, dyspepsia, nausea, vomiting, abdominal pain, blurred vision, priapism, erectile difficulties, ejaculatory weakness, indifference, urinary incontinence, rhinitis, rash and other allergic reactions. 3, may cause extrapyramidal symptoms, such as: muscle tension, tremor, rigidity, flow, bradykinesia, akathisia and acute dystonia, by reducing the dose or giving anti Parkinson's syndrome drugs can be eliminated.
4. Occasionally, symptoms of (orthostatic) hypotension, (reflex) tachycardia, or hypertension may occur.
There is weight gain, edema, and elevated levels of liver enzymes. 5,
6. In foreign clinical studies, it was reported that the incidence of cerebrovascular adverse events, such as stroke, transient ischemic attack, including death events, in elderly patients (average age 85 years) with dementia-related psychiatric symptoms treated with risperidone tablets was significantly higher than that of placebo.
7. Elderly patients with dementia-related psychiatric symptoms may have an increased risk of cerebrovascular adverse events when using this product, which should be noted.
8, occasionally due to the patient's polydipsia or antidiuretic hormone secretion disorder (SIADH) caused by water poisoning.
9, will cause an increase in plasma prolactin concentration, its related symptoms are: galactorrhea male female breast, menstrual disorders, amenorrhea.
10. Occasional tardive dyskinesia, malignant syndrome, temperature disorders and epilepsy
Make.
11. Mild decrease in neutrophil and/or platelet counts has been reported.
taboo]
Disallowed in patients with known hypersensitivity to risperidone, paliperidone, or excipients in this product
[Note]]
1. Alzheimer's patients
1.1 total mortality
A pooled analysis of 17 controlled trials of several atypical antipsychotics, including this product, showed an increase in mortality in patients with dementia in the atypical antipsychotic group compared with the placebo group. In a placebo-controlled trial of this product in this population, the mortality rate was 4.0 per cent in the drug group and 3.1 per cent in the drug group. The average age of those who died was 86 years (range, 67-100 years).
1.2 in combination with furosemide
In a placebo-controlled study of this product in patients with senile dementia, the mortality rate of patients with risperidone combined with furosemide was higher than that of patients with risperidone or furosemide alone, 7.3%(mean age 89 years, range 75-97 years), 3.1%(mean age 84 years, range 70-96 years), and 4.1%(mean age 80 years, range 67-90 years), respectively. In two of the four clinical trials, increased mortality was observed in patients who were co-administered with Semito.
Although no clear pathophysiological mechanism has been identified to explain this phenomenon, and the causes of death vary among patients, caution should be exercised in assessing the risk benefit of concomitant administration of risperidone and semide in older patients. In patients taking risperidone in combination with other diuretics, there was no such increase in mortality. Because dehydration is a very important cause of death in patients with senile dementia, so should try to avoid the occurrence of dehydration.
2. Cerebrovascular accident (CAE)
In a placebo-controlled study of elderly patients (mean age 85 years, range 73-97 years), a higher incidence of adverse cerebrovascular events (cerebrovascular accident and transient ischemic attack), including death, was observed in the risperidone group than in the placebo group.
3. orthostatic hypotension
Due to its blocking effect on emetic receptors, orthostatic hypotension may occur, especially during the initial dose adjustment phase of treatment. Clinically significant hypotension has been observed with post-marketing concomitant antihypertensive drugs. For patients with known cardiovascular disease (such as heart failure, myocardial infarction, conduction abnormalities, dehydration, low blood volume or cerebrovascular disease) should be used with caution, the dose should be gradually increased according to the recommend dose (see 【Dosage】), if the phenomenon of low blood pressure, should consider reducing the dose.
4, Leukopenia, neutropenia and agranulocytosis Events of leukopenia, neutropenia and agranulocytosis have been reported with antipsychotic drugs (including risperidone). During post-marketing surveillance, granulocytes
Reports of deficiency are very rare (<1/10,000 patients). Patients with a history of significant leukopenia or drug-induced leukopenia/neutropenia should be monitored during the first few months of treatment. In the absence of other precipitating factors, discontinuation of this product should be considered once significant leukopenia is found.
Patients with clinically significant neutropenia should be carefully monitored for symptoms or signs of fever or other infection, and should be treated immediately if these symptoms or signs occur. For patients with severe neutropenia (absolute neutrophil count <1x10 9/L), the use of this product should be stopped, and follow up to monitor the white blood cell count until it returns to normal.
5, Venous Thromboembolism (VTE)
Cases of venous thromboembolism have been reported using antipsychotics. Since risk factors for VTE are often present in patients treated with antipsychotics, all possible risk factors for VTE should be judged before and during treatment with Zipin, and preventive measures should be taken.
6. Tardive Dyskinesia/Extrapyramidal Symptoms (TD/EPS)
Similar to all other drugs with dopamine receptor antagonist properties, this product may also cause tardive dyskinesia, which is characterized by rhythmic involuntary movements, mainly on the tongue and face. There are reports that the occurrence of extrapyramidal symptoms is a risk factor for the development of tardive dyskinesia, and compared with other traditional antipsychotic drugs, this product is less likely to cause extrapyramidal symptoms, so compared with traditional antipsychotic drugs, This product has a lower risk of causing tardive dyskinesia. If symptoms of tardive dyskinesia occur, discontinuation of all antipsychotics should be considered.
Extrapyramidal symptoms and psychostimulants-Patients receiving a combination of psychostimulants (e. g., methylphenidate) and risperidone should be cautious because extrapyramidal symptoms may occur when one or both drugs are adjusted. Gradual discontinuation of one or both therapeutic agents should be considered (see section Drug Interactions).
7. Neuroleptic Malignant Syndrome (NMS)
There have been reports of neuroblocker malignant syndrome, characterized by high fever, muscle rigidity, autonomic instability, altered consciousness, and elevated serum creatine phosphokinase levels, and possibly myoglobinuria (rhabdomyolysis) and acute renal failure, as well as administration of traditional antipsychotics. At this time, all antipsychotic medications, including this product, should be discontinued.
8, Parkinson's disease or Lewy body crazy fruit
In patients with Lewy body dementia or Parkinson's disease, the benefit of the prescription of antipsychotics (including this product) should be weighed, which may increase the risk of neuroleptic malignant syndrome. At the same time, the sensitivity of the above population to antipsychotic drugs increased, in addition to the emergence of extrapyramidal symptoms, there will be confusion, dullness, unstable position with frequent falls.
9, High blood sugar and diabetes
During the use of this product, there have been reports of hyperglycemia, diabetes mellitus and aggravation of pre-existing diabetes mellitus. The inherent high risk of diabetes in schizophrenia and the increasing incidence of diabetes in the normal population complicate the assessment of the association between the use of atypical antipsychotics and glucose abnormalities. Because of these complicating factors, the relationship between the use of atypical antipsychotics and adverse events related to hyperglycemia is not well understood, and patients treated with atypical antipsychotics, including this product, should be monitored for symptoms of hyperglycemia and diabetes (see section [Adverse Reactions]).
10. Dyslipidemia
Dyslipidemia is now observed in patients treated with atypical antipsychotics
化
11. Weight Gain Significant weight gain has been reported. When using this product, weight monitoring should be carried out, 12, hyperprolactinemia
As with other drugs that antagonize the dopamine D2 receptor, prolactin levels are elevated with this product and can persist during long-term administration. Prolactin levels are increased to a greater extent than other antipsychotic drugs.
13, fall.
Sleep, orthostatic hypotension, movement, and sensory instability have been reported with antipsychotics (including this product), leading to falls and consequent fractures or other fall-related injuries. For patients with diseases, conditions, or medications that may exacerbate such effects (especially the elderly), the risk of falls should be assessed at the beginning of antipsychotic therapy, patients on long-term antipsychotic therapy were evaluated repeatedly. 14. Potential cognitive and movement disorders
Drowsiness is a common adverse reaction associated with the treatment with RETIN, especially when the patient is asked directly for confirmation. This adverse reaction is dose-related. In the study using the checklist to detect adverse events, 41% of patients in the high-dose group (16mg/day) reported sleep, while 16% of patients in the placebo group reported sleep. Direct questioning was more sensitive than spontaneous reporting when detecting adverse events, with sleepiness reported in 8% of patients in the 16mq/day dose group and 1% in the placebo group. Because this product may affect judgment, thinking or motor skills, patients should not operate dangerous machinery (including driving a car) until it is reasonably certain that treatment with this product will not cause adverse effects.
14. Potential cognitive and movement disorders
Drowsiness is a common adverse reaction associated with the treatment with RETIN, especially when the patient is asked directly for confirmation. This adverse reaction is dose-related. In the study using the checklist to detect adverse events, 41% of the patients in the high-dose group (16mg/day) reported drowsiness, while 16% of the patients in the drug group reported drowsiness. Direct questioning was more sensitive than spontaneous reporting in detecting adverse events, with sleepiness reported in 8% of patients in the 16mg/day dose group and 1% of patients in the placebo group. Because this product may affect judgment, thinking or motor skills, fools should not operate dangerous machinery (including driving a car) until it is reasonably certain that the treatment with this product will not cause adverse effects.
15. Difficulty swallowing
Esophageal motility disorders and aspiration are known to be associated with antipsychotic drug use, and aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer's dementia. Patients at risk of aspiration pneumonia should use this drug and other antipsychotic drugs with caution.
16, Intraoperative Iris Relaxation Syndrome (IFIS)
Intraoperative iris relaxation syndrome was observed during cataract surgery in those who used drugs with a1a-adrenoceptor antagonistic effect (including this product).
IFIS may increase the risk of intraoperative and postoperative ocular complications, and patients should inform their ophthalmologist of current or previous use of drugs with q1a-adrenoceptor antagonism prior to surgery. The potential benefit of discontinuing g1-blocking therapy before cataract surgery has not been established, and the risks of discontinuing antipsychotic drug therapy must be weighed.
17. QT interval
As with other antipsychotic drugs, caution should be exercised when administering this product to patients with a history of cardiac arrhythmias, congenital long QT syndrome, and when combined with drugs known to prolong the QT interval,
18, Priapism Drugs with an adrenergic blocking effect have been reported to cause priapism. During the post-marketing surveillance period, there have been reports of abnormal penile vigor (see [Adverse Reactions]).
19, Thermoregulation
The use of antipsychotics can impair the body's ability to lower deep body temperature. Appropriate care is recommended for those who use this product when the patient is in conditions that may increase deep body temperature (such as strenuous exercise, being in a high temperature environment, receiving combined treatment with anticholinergic active drugs, or suffering from dehydration),
20, antiemetic effect
Antiemetic effects have been observed in preclinical studies with risperidone. This effect, if it occurs in humans, may mask the signs and symptoms of certain drug overdoses or diseases, such as blocked intestines, Raytheon syndrome, and brain tumors.
21. Seizures
As with other antipsychotics, caution should be used in patients with a history of seizures or other conditions that could potentially lower the seizure threshold.
22 and others.
For specific dosages recommend in elderly patients, patients with hepatic or renal impairment, or patients with Alzheimer's disease, see the sections [Geriatric use] and [Administration and dosage].
This product has an effect on activities that require alertness. Therefore, it is recommended that patients should not drive cars or machines during treatment until their sensitivity to this product is known.
Please keep it easy for children to take everywhere,
Medication for pregnant and lactating women]
It is not known whether this product is safe for pregnant women.
A retrospective observational cohort study based on the US Claims database compared the risk of congenital craggy in live births to women with and without antipsychotic medication during early pregnancy. After adjusting for existing confounding variables in the database, the risperidone group had an increased risk of congenital malformations compared with the group not exposed to antipsychotic drugs (relative risk = 1.26,95: 1.02-1.56). No biological mechanism has been found to explain this finding, and no teratogenic effects have been observed in non-clinical studies. Based on the results of this observational study, a causal relationship between in utero exposure to risperidone and congenital malformations cannot be established.
Animal experiments have shown that risperidone has no direct toxicity to reproduction, and only some indirect prolactin and central nervous system-mediated effects have been observed,
Fetuses exposed to antipsychotic drugs (including risperidone) during the third trimester of pregnancy are at risk of developing extrapyramidal symptoms or withdrawal symptoms after birth, which may vary in severity. These symptoms include agitation, hypertonia, hypotonia, tremor, lethargy, respiratory distress, and feeding disorders.
Use during pregnancy only if the benefits outweigh the risks,
Lactation: Animal tests have shown that risperidone and 9-hydroxy-risperidone are excreted in the milk of animals. At the same time, human trials have also shown that this product is excreted through breast milk, so women taking this product should not breastfeed.
children medication]
See [Indications] and [Usage and Dosage].
elderly medication]
The recommended starting dose is 0.5mg twice daily, and the dose can be adjusted according to individual needs. Dose increases can range from 0.5mg twice daily to 1 to 2mg twice daily.
pharmacodynamic interaction
Drugs and alcohol acting on the central nervous system In view of the main effects of this product on the CNS, caution should be used in combination with other central nervous system drugs or alcohol.
Levodopa and dopamine agonists
This product may antagonize the effects of levodopa and other dopamine agonists,
Drugs that cause low blood pressure
Clinically significant hypo-
Pressure
Drugs that prolong the QT interval
Caution should be exercised when simultaneously prescribing this product and drugs that prolong the QT interval,
pharmacokinetic correlation interaction
Food does not affect the absorption of this product.
Risperidone is mainly metabolized by CYP2D6, and a small part is metabolized by CYP3A4. Risperidone and its active metabolite 9-hydroxy-risperidone are both substrates of P-glycoprotein (P-gp). Substrates that alter CYP2D6 activity or substrates that strongly inhibit/induce CYP3A4 and/or P-gp activity can affect the pharmacokinetics of the antipsychotic active components of risperidone.
Potent CYP2D6 inhibitors
Concomitant treatment with a potent CYP2D6 inhibitor may increase plasma concentrations of risperidone alone, but less so of the antipsychotic component of risperidone. Higher doses of a potent CYP2D6 inhibitor may increase the concentration of the antipsychotic component of risperidone (eg, paroxetine, the same below). When starting or discontinuing concomitant use of paroxetine or other potent CYP2D6 inhibitors, especially at higher doses, physicians should reevaluate the product dose.
CYP3A4 and/or P-gp inhibitors
Concomitant administration with potent CYP3A4 and/or P-gp inhibitors significantly increases plasma concentrations of the antipsychotic active ingredient of risperidone. Physicians should reevaluate the dose of risperidone when initiating or discontinuing concomitant use of itraconazole or other potent CYP3A4 and/or P-gp inhibitors.
CYP3A4 and/or P-gp Inducers Concomitant administration with potent CYP3A4 and/or P-gp inducers may reduce plasma concentrations of the antipsychotic active ingredient of risperidone. Physicians should reevaluate this product when starting or discontinuing concomitant use of carbamazepine or other potent CYP3A4 and/or P-gp inducers.
Drugs that are highly protein-bound
When combined with drugs with high protein binding, no clinically relevant plasma protein exchange occurred.
When using concomitant drugs, the corresponding instructions should be read for information on metabolic pathways and possible dosage adjustments.
Pediatric population
Interaction studies were conducted in adults only. The relevance of the findings to pediatric patients is unclear,
Examples
Drugs that may cause potential interactions or not interact with risperidone are listed below:
Antibacterial drugs:
. The pharmacokinetics of erythromycin, a moderate CYP3A4 inhibitor, risperidone alone, and the antipsychotic active ingredient of risperidone were unchanged,
. Rifampicin, a potent CYP3A4 inducer and P-gP inducer, decreased plasma concentrations of the antipsychotic active component of risperidone.
anticholinergic cool portuguese:
· Dopiperzil and galantamine, both CYP2D6 and CYP3A4 substrates, have no clinically relevant effects on the pharmacokinetics of risperidone alone and the antipsychotic active ingredient of risperidone.
Antiepileptic drugs:
. Carbamazepine, a potent CYP3A4 inducer and P-gp inducer, reduces plasma levels of the antipsychotic active component of risperidone,
· Topiramate slightly decreased the bioavailability of the risperidone single product, but did not decrease the bioavailability of the antipsychotic active component of risperidone. Therefore, this interaction is unlikely to have clinical significance.
· No clinically relevant effect of risperidone on the pharmacokinetics of valproate or topiramate,
Antifungal drugs:
Itraconazole, a potent CYP3A4 and P-gp inhibitor, increases the plasma concentration of the antipsychotic active ingredient of risperidone by about 70% at a dose of 200mg/day when risperidone is 2 to 8mg/day.
. Ketoconazole, a potent CYP3A4 and P-gp inhibitor, increased plasma concentrations of risperidone and decreased plasma concentrations of 9-hydroxy-risperidone at a dose of 200mg/day.
Antipsychotics:
· Phenothiazines, which may increase plasma concentrations of ripeginone, but not
Plasma concentrations of the antipsychotic active component of the tone ketone.. Aripiprazole, a CYP2D6 and CYP3A4 substrate: Risperidone tablets or injections do not affect the overall pharmacokinetics of aripiprazole and its active metabolite, dehydroaripiprazole.
· Fluorazepine: long-term combination of risperidone and oxyazepine can reduce the clearance rate of risperidone
Antivirals: • Protease inhibitors: No formal study data are available; however, because ritonavir is a potent CYP3A4 inhibitor and a weak CYP2D6 inhibitor, ritonavir and ritonavir potentiating protease inhibitors may increase the concentration of the antipsychotic active ingredient of risperidone.
B- Blocker:
· Some B- blockers may increase the blood concentration of risperidone single product, but not the plasma concentration of the antipsychotic active component of risperidone.
calcium channel blockers:
● Verapamil. A moderate CYP3A4 inhibitor and P-gp inhibitor that increases plasma concentrations of risperidone single product and the antipsychotic active components of risperidone.
Digitalis Glycosides:
· No clinically relevant effect of risperidone on the pharmacokinetics of digoxin,
Diuretics:
· Gisemide: About increased mortality in elderly patients with dementia in concomitant use of furosemide (see 【 Precautions 】).
Gastrointestinal drugs:
· H2-receptor antagonists: Cimetidine and ranitidine, both weak inhibitors of CYP2D6 and CYP3A4, increased the bioavailability of the risperidone single product, but only slightly increased the bioavailability of the antipsychotic active component of risperidone.
Lithium:
● There was no clinically relevant effect of risperidone on the pharmacokinetics of lithium.
SSRIs and tricyclic antidepressants:
Fluoxetine, a potent CYP2D6 inhibitor, increased plasma concentrations of the risperidone single product, but less so of the antipsychotic active component of risperidone.
● Paroxetine, a potent CYP2D6 inhibitor, increased plasma concentrations of the risperidone single product, whereas doses up to 20mg/day did not increase plasma concentrations of the antipsychotic active component of risperidone. However, higher doses of paroxetine may increase the concentration of the antipsychotic active ingredient of risperidone.
· Tricyclic antidepressants may increase plasma concentrations of risperidone alone, but not the antipsychotic active component of risperidone. Amitriptyline does not affect the pharmacokinetics of risperidone or the antipsychotic active ingredient.
Sertraline (a weak CYP2D6 inhibitor) and fluvoxamine (a weak CYP3A4 inhibitor) at doses up to 100mg/day did not cause clinically significant changes in the concentration of the antipsychotic active ingredient of risperidone. However, when the dose of sertraline or fluvoxamine is higher than 100mg/day, the concentration of the antipsychotic active ingredient of risperidone can be increased.
drug overdose]
Signs and Symptoms
When symptoms of an acute overdose occur, consideration should be given to the presence or absence of other factors that may be associated with the use of other drugs.
In general, the reported signs and symptoms of overdose are due to an extension of its pharmacological effects, including somnolence and sedation, tachycardia and hypotension, and extrapyramidal symptoms. There have been reports of QT interval prolongation and convulsions at the time of drug overdose. There have been reports of torsades de pointes when an overdose of this product was combined with paroxetine.
Processing
In overdose rescue, airway patency should be maintained, adequate oxygen and good ventilation should be ensured, activated charcoal and laxatives should be considered, and immediate cardiovascular monitoring, including continuous electrocardiographic monitoring, should be performed to detect possible arrhythmias. There is no specific antidote to this product, therefore, appropriate supportive therapy should be used. Hypotension and circulatory collapse can be corrected with appropriate measures such as intravenous fluids or the administration of sympathomimetics. Once severe extrapyramidal symptoms appear, anticholinergic drugs should be given, and close medical monitoring and supervision should be continued until the patient recovers.
Abuse
The potential abuse of risperidone has not been systematically studied in animals or humans. Although clinical trials have not shown a tendency to seek behavior, such observations are not systematic and cannot be used to predict post-marketing misuse, diversion, and/or abuse of CNS-active drugs based on this limited experience. Therefore, patients with a history of drug abuse should be evaluated with caution and closely observed for signs of misuse or abuse of risperidone (eg, development of tolerance, dose escalation, drug-taking behavior). Dependency
Potential resistance or somatic resistance to risperidone has not been systematically studied in animals or humans
Put
clinical trial]
1. Schizophrenia
Touching
short-term efficacy
The efficacy of risperidone in the treatment of schizophrenia was demonstrated in 4 short-term (4-to 8-week) controlled trials of psychiatric inpatients who met criteria for DSM-1I1-R schizophrenia
In these studies, several scale instruments were used to assess psychiatric signs and symptoms, among them the Brief Psychiatric Rating Scale (BPRS), a generic psychopathology multi-item scale traditionally used to evaluate the effects of pharmacologic treatment for schizophrenia. The BPRS psychotic cluster (conceptual confusion, hallucinatory behavior, paranoia, and abnormal thinking) is a particularly useful subset for assessing patients with active schizophrenia. The second traditional assessment is clinical global impression (CG1), which can reflect the impression of a skilled observer (familiar with the manifestations of schizophrenia) on the patient's overall clinical status. In addition, the Positive and Negative Symptom Scale (PANSS) and the Negative Symptom Rating Scale (SANS) were used.
The results were as follows:(1) In a 6-week placebo-controlled trial (n = 160) in which risperidone was titrated to doses of up to 10mg/day (twice daily), risperidone was generally superior to placebo in BPRS total scores as well as in BPRS psychotic clusters, and slightly superior to Ansanga in SANS.
(2) In an 8-week controlled trial (n = 513) using four fixed doses of risperidone (2mg/day, 6mg/day, 10mg/day, and 16mg/day twice daily), all four risperidone groups outperformed placebo in terms of BPRS total score, BPRS psychotic cluster, and CGI severity scores: the three highest-dose risperidone groups were superior to the placebo group in the PANSS-negative subscale. The most consistent positive response for all indicators was seen at the 6mg dose, and no increase in benefit was seen at higher doses,
(3) In an 8-week dose comparison test (n = 1356), five round doses of risperidone (1mgv day, 4mg/day, 8mg/day, 12mg/day and 16mg/day, twice a day) were used. In terms of BPRS total score, BPRS psychiatric cluster and CGI severity score, the 4 higher dose risperidone groups were better than 1mg risperidone dose groups. In the PANSS subscale, no dose group was better than the 1mg group, and the most consistent positive response was observed in the 4mg dose group.
(4) In a 4-week placebo-controlled dose comparison test (n = 246), two fixed doses of risperidone (4 and 8mg/day, once a day) were used. The two riperidone negative groups were superior to the placebo group in several PANSS indexes such as curative effect index (PANSS total score reduction> 20%), PANSS total score, BPRS psychosis cluster (derived from PANSS), etc. The results of the 8mg dose group were generally better than the 4mg dose group.
Long-term efficacy
In a long-term trial, 365 adult outpatients who met the criteria for DSM-IV schizophrenia and were clinically stable for at least 4 weeks on antipsychotic medication were randomly assigned to risperidone (2 to 8mg/day) or to the active comparator group and were observed for relapse over 1 to 2 years. During this period, the time to relapse was significantly longer in patients receiving risperidone compared to those receiving the active comparator.
Pediatrics
The efficacy of risperidone for the treatment of schizophrenia in adolescents aged 13 to 17 years was demonstrated in two short-term (6-week and 8-week) double-blind controlled trials. All patients met the diagnostic criteria for DSM-IV schizophrenia and had an acute attack when enrolled. In the first trial (study #1), patients were randomly assigned to one of three treatment groups: risperidone 1-3mg/day (n = 55, mean modal dose = 2.6mg); risperidone 4-6mg/day (n = 51, mean modal dose = 5.3mmg) or placebo (n = 54). In the second trial (study #2), patients were randomly assigned to risperidone 0.15 to 0.6mg/day (n = 132, mean modal dose = 0.5mg) or risperidone 1.5 to 6mg/day (n = 125, mean modal dose = 4mg). In all cases, the starting dose of study drug was 0.5mg/day (except for the 0.15-0.6mg/day dose arm of Study #2, where the starting dose was 0.05mg/day) and titrated to the target dose range on approximately Day 7. Subsequently, by day 14, the dose was increased to the maximum tolerated dose within the target dose range. The primary efficacy variable in all studies was the change from baseline in the PANSS total score.
The results demonstrate the efficacy of all doses of risperidone (1-6mg/day) compared with placebo, as measured by the reduction in the total PANSS score. The efficacy of the major parameters for the 1-3mg/day dose group was similar to the 4-6mg/day dose group in Study #1 and was similar to the efficacy demonstrated for the 1.5-6mg/day dose group in Study #2. In Study #2, the efficacy of the 1.5-6mg/day dose group was statistically significantly greater than the efficacy of the 0,15-0.6mg/day dose group. At doses above 3mg/day, no increasing trend was seen.
2. Bipolar Ankle-Monotherapy
Adult
The efficacy of risperidone for the DSM-IV of acute manic or mixed episodes was demonstrated in two short-term (3-week) controlled trials of antipsychotics in adults who met the criteria for treatment of acute manic or mixed episodes in bipolar I disorder, including patients with and without psychotic features.
The primary scale used to assess mania symptoms in these trials is the Young's Mania Scale (YMRS), an 11-item clinical rating scale traditionally used to assess the degree of mania symptoms (irritability, disruptive/aggressive behavior, sleep, heightened mood, language, increased activity, sexual interest, language/thought disorders, thought content, imagery, and insight), scores ranged from 0 (no manic traits) to 60 (highest score). The primary outcome of these trials was the change from baseline in the YMRS total score, which was as follows:
(1) In a 3-week placebo-controlled trial (n = 246) limited to manic patients, risperidone was superior to placebo in reducing YMRS total score in doses ranging from 1 to 6mg/day, with a starting dose of 3mg/day (mean modal dose of 4.1mg/day).
(2) In another 3-week placebo-controlled trial (n = 286), involving a dose range of 1 to 6mg/day, once a day, with an initial dose of 3mg/day (mean modal dose of 5.6mg/day), risperidone was superior to placebo in reducing the total score of YMRS.
Pediatrics
In a 3-week, randomized, double-blind, placebo-controlled multicenter trial, the efficacy of risperidone in the treatment of mania in children or adolescents with type I bipolar disorder was confirmed. The trial included patients aged 10 to 17 years with acute manic or mixed episodes of type I bipolar disorder. Patients were randomly assigned to one of three treatment groups: risperidone 0.5-2.5mg/day (n = 50, mean modal dose = 1.9mg); risperidone 3-6mg/day (n = 61, mean modal dose = 4.7mg); or placebo (n = 58). In all cases, the starting dose of study drug was 0.5mg/day and was titrated to the target dose range on approximately Day 7, with further dose escalation to the maximum tolerated dose within the target dose range on Day 10. The primary scoring tool used to assess efficacy in this study was the change from baseline in the YMRS total score.
The results of this study demonstrate the efficacy of both doses of risperidone compared with placebo, as measured by a significant reduction in the total YMRS score. The main parameters of the 3-6mg/day dose group were similar to the 0.5-2.5mg/day dose group. At doses above 2.5mg/day, no trend toward increased efficacy was seen.
3. Bipolar mania-as adjunctive therapy in combination with salt or valproate The efficacy of risperidone in combination with lithium or valproate in the treatment of acute manic or mixed episodes was demonstrated in a controlled trial in adults who met the DSM-IV criteria for bipolar I disorder. Patients with or without psychotic features and with or without a rapid cycling course were included in the trial.
(1) In this 3-week, placebo-controlled, combination trial, 148 inpatients or outpatients whose symptoms of mania or mixed episodes were not adequately controlled by lithium or valproate were randomly assigned to receive risperidone, placebo, or an active control (in combination with their respective original treatment). Risperidone (dose range 1 to 6mg/day, starting at 2mg/day, mean modal dose 3.8mg/day) combined with lithium or propionate (therapeutic dose range 0.6mEq/L to 1,4mEq/L; or 50mcg/mL to 120mcg/mL) was superior to lithium or valproate monotherapy in reducing YMRS total score.
(2) In a second 3-week, placebo-controlled, combination trial, 142 inpatients or outpatients whose symptoms of mania or mixed episodes were not adequately controlled by lithium or valproate were randomly assigned to receive risperidone or placebo (in combination with their respective original treatment). In terms of reducing YMRS total score, risperidone (dose range: 1-6mg/day, starting dose: 2mg/day, mean modal dose: 3.7mg/day) was combined with lithium, valproate or carbamazepine (therapeutic dose range: lithium 0.6mEq/L to 1.4mEq/L; valproate 50mcg/mL to 125mcg/mL, respectively; carbamazepine 4 ~ 12mcg/mL) is not superior to lithium, valproate or carbamazepine monotherapy. The possible reason for the failure of this trial was carbamazepine-induced clearance of risperidone and 9-hydroxy-risperidone, which resulted in lower than therapeutic levels of risperidone and 9-hydroxy-risperidone.
4. Autism-related irritability
short-term efficacy
The efficacy of risperidone for the treatment of autism-related irritability was demonstrated in two 8-week placebo-controlled trials in children and adolescents (5 to 16 years of age) who met criteria for DSM-IV autism. More than 90% of the subjects were under 12 years of age, with most weighing
More than 20kg(16~104.3kg). Efficacy was assessed using two assessment scales: the Abnormal Behavior Checklist (ABC) and the Clinical Global Impression-Change (CGI-C) scale. The primary outcome measure in both trials was the change from baseline to endpoint on the ABC irritability subscale (ABC-I). ABC-I subscale measures the emotional and behavioral symptoms of autism, including symptoms such as aggression, intentional self-harm, bad temper and rapid mood changes. In one study, the CGIC score at endpoint was the composite primary outcome measure.
The results of these tests were as follows:
(1) In an 8-week placebo-controlled trial, autistic children and adolescents (n = 101) aged 5 to 16 years received twice-daily doses of placebo or risperidone 0.5 to 3.5mg/day (adjusted for body weight). Risperidone was started at 0.25mg/day or 0.5mg/day, depending on baseline body weight (<20kg and 220kg), and titrated to a clinically effective dose (mean modal dose of 1.9mg/day, equivalent to 0.06 mg/kg day); compared with placebo, ABC-1 subscale and CGI-C scale ratings were significantly improved.
(2) In another 8-week placebo-controlled trial, autistic children aged 5 to 12 years (n = 55) received risperidone 0.02 to 0.06 mg/kg/day, once or twice daily, with a starting dose of 0.01 mg/kg/day, and titrated to a clinically effective dose (mean modal dose of 0.05 mg/kg/day, equivalent to 1,4mg/day); compared with the drug, the ABC-1 subscale score was significantly improved.
The third trial was a 6-week, multicenter, randomized, two-word, placebo-controlled, fixed-dose study evaluating the efficacy and safety of less than recommend doses of risperidone in subjects aged 5 to 17 years (N = 96) with autism (defined by DSM-IV criteria) and irritability and related behavioral symptoms. Approximately 77% of patients were younger than 12 years of age (mean age = 9 years) and 88% were male. Most patients (73%) weighed less than 45kg (mean weight = 40kg). About 90% of patients had not used antipsychotic drugs before entering the study,
For risperidone, there are 2 fixed doses (high dose and low dose) based on body weight. For patients weighing 20 to <45kg, the high dose is 1.25mg per day; for patients weighing 245kg, the high dose is 1.75mg per day; for patients weighing 20 to <45kg, the low dose is 0.125mg per day; for patients weighing 245kg, the low dose is 0.175mg per day. Once daily dosing was performed in the morning and in the evening if sedation occurred.
The primary efficacy endpoint was the mean change from baseline to the end of Week 6 in the abnormal behavior checklist-irritation subscale (ABC-I) score. The study demonstrated the efficacy of high doses of lipeb-measured by the mean change in ABC-I score. The efficacy of low-dose risperidone has not been demonstrated. The mean baseline ABC-I score was 29 in the placebo group (n-35): 27 in the low-dose risperidone group (n = 30) and 28 in the high-dose risperidone group (n = 31). Mean changes in ABC-I scores were -3.5, 7.4, and -12.4 in the placebo, low-dose, and high-dose groups, respectively, with results reaching statistical significance in the high-dose group (p <0.001) but not in the low-dose group (p = 0.164).
Long-term efficacy
After the completion of the first 8-week double-talk study, 63 patients entered the open-label study extension period and received risperidone for 4 or 6 months (depending on whether the patients received risperidone or placebo in the double-blind study). During this open-label treatment period, patients maintained a mean modality dose of 1.8-2.1mg/day (equivalent to 0.05-0.07 mg/kg/day).
Patients who maintained a positive response to risperidone (defined as a 25% improvement on the ABC-I subscale and a CGIC rating of "substantial improvement" or "very substantial improvement") during the 4-to 6-month open-label treatment period (average of approximately 140 days) continued to be randomized to risperidone or placebo in the 8-week double-blind withdrawal study (n = 39 of 63 patients). A pre-planned interim analysis of data from individuals who completed the withdrawal study (n = 32) by an independent data safety monitoring committee demonstrated a significant reduction in relapse rates in the Lipede group compared with the placebo group. Based on the interim analysis, the study was terminated because a statistically significant effect on relapse prevention had been demonstrated. Relapse was defined as a 25% deterioration in the latest assessment of the ABC1 subscale (compared to baseline in the random withdrawal phase).
5. Behavioral Disorders
Pediatrics
The efficacy of risperidone for short-term treatment of disruptive behavior was demonstrated in two double-rich placebo-controlled studies of 228 patients aged 5 to 12 years with a DMS-IV diagnosis of disruptive behavior disorder (DBD) and borderline intellectual functioning, or mild or middle-seated mental retardation/learning disability. in both studies. Risperidone at 0.02 to 0.06 mg/kg/day was significantly better than placebo for the prespecified primary end point (I. e., change from baseline in the behavioral problems subscale of the Nisonger Children's Behavior Rating Scale (N-CBRF) at week 6).
Pharmacological Poison Lu]
Pharmacological action
Risperidone is a selective monoaminergic antagonist, with high affinity for 5HT2 receptor, D2 receptor, 1 and 2 receptor and H1 receptor. Low to moderate binding force for SHT1C, 5HT1D and 5HT1A, weak affinity for D1 and droperidol-sensitive early receptors, and no affinity for M receptors or Pin 1 and Pin 2 receptors.
Risperidone is the same as other drugs used to treat schizophrenia. The mechanism for the treatment of schizophrenia is unclear, and it is thought that the therapeutic effect is the result of a combined effect of antagonism of D2 receptors and 5HT2 receptors. Antagonism of receptors other than D2 and 5HT2 may be related to other effects of risperidone.
Toxic compliance research
Genotoxicity
The risperidone Ames test, the mouse lymphoma test, the in vitro rat hepatocyte DNA repair test, the in vivo micronucleus test in mice, the Drosophila sex-associated recessive lethal test, and the in vitro human lymphocyte or Chinese hamster fine chest chromosome transmutation test were all negative.
reproductive toxicity
In three W/istar rat reproductive toxicity tests (two fertility and early embryo developmental toxicity tests and one multi-generation reproductive toxicity test), risperidone was administered orally at 0.16 to 5 mg/kg [in mg/m2, 0.1 to 3 times the maximum recommended human dose (MRHD) of 16mgv days (see [Administration and Dosage]). Affects mating behavior, but does not affect biological business. This effect occurred only in male rats, as the effect on mating behavior was observed in fertility and early embryo development tests administered only to male rats. Sub chronic fertility test in Beagle dogs. When risperidone is administered orally at a dose of 0.31 to 5 mg/kg (in mg/m2, 0.6 to 10 times the MRHD). Sperm motility and concentration decreased, and serum testosterone level decreased dose-related with the same dose. After drug withdrawal, serum testosterone level and sperm parameters could be partially restored, but still at a low level. Rat
No effect dose was determined in either dog or dog. Risperidone was administered orally at doses of 0.63-10 mg/kg and 0.31-5 mg/kg (0.4-6 times and 0.4-6 times MRHD in mg/m2, respectively) in SD and Wistar rats and New Zealand rats, and no induced effect was observed.
In three rat reproductive toxicity tests (two perinatal toxicity tests and one multigenerational reproductive toxicity test), when risperidone was administered orally at a dose of 0.16-5 mg/kg (0.1-3 times MRHD in mg/m2, respectively), the mortality of young rats increased in the first 4 days of lactation. It is not clear whether these deaths are due to direct effects on the fetus or young, or to effects on the mother. A no-effect dose for increased pup mortality in rats was not determined. In a perinatal toxicity trial, stillbirths increased in rat pups at 2.5 mg/kg (1.5 times MRHD in mg/m2). In a cross-rearing test in rats, the toxic effects on the fetus or pups were manifested by a decrease in the number of live pups at birth, an increase in the number of dead pups, a decrease in the birth weight of pups administered to mothers, and an increase in the death of pups administered to mothers on the first day of birth, regardless of whether the pups were cross-reared. Risperidone may be detrimental to maternal behavior, with reduced weight gain and survival (days 1 to 4 of lactation) in pups produced in control animals and raised by dosing mothers. These effects were all observed at a dose of 5 mg/kg (3 times MRHD in mg/m2).
Risperidone can be transported to rat pups through the placenta.
Carcinogenicity
Mice and rats were given risperidone 0.63, 2.5 and 10 mg/kg (2, 9 and 38 times of MRHD in mg/kg respectively: 0.2, 0.75 and 3 times of MRHD in mg/m2 in mice and 0.4.1.5 and 6 times of MRHD in rats respectively), and the administration period was 18 months and 25 months respectively. mice did not reach the maximum tolerated dose. The results showed a statistically significant increase in pituitary adenomas in female mice, pancreatic endocrine adenomas in male rats, and breast cancer in female mice and male and female rats.
Antipsychotic drugs can cause long-term elevation of prolactin levels in rodents. Prolactin levels were not measured in the risperidone carcinogenicity test, but in the subchronic toxicity test, oral administration of risperidone at the same dose as in the carcinogenicity test resulted in a dose-dependent increase of prolactin levels in mice and rats, up to 5 to 6 times, respectively. When other antipsychotic drugs are administered chronically, an increase in the incidence of pituitary, endocrine pancreatic, and breast tumors has been found in rodents and is thought to be mediated by prolactin. The correlation between the occurrence of prolactin-mediated endocrine tumors in rodents and the risk of human use is unclear.
Other Toxicity
In the 40-week toxicity test at young age, a decrease in bone length and density was observed when risperidone was given orally at 0.31, 1.25, and 5 mg/kg/day, with a no-effect dose of 0.31 mg/kg/day. In addition, a delay in sexual maturation was observed for both male and female animals in all dose groups. After a recovery period of 12 weeks from drug withdrawal, the above effects on female animals showed no or substantially no reversibility.
In the toxicity test of young rats, when risperidone was given orally from 12 to 50 days of age, reversible learning and memory impairment was observed only in sexual animals, and the non-affecting dose was 0.63 mg/kg/day. No effects on neurobehavioral or reproductive development were seen at the highest dose of 1.25 mg/kg/day.
pharmacokinetics]
Risperidone can be completely absorbed after oral administration and reach the peak blood concentration within 1 to 2 hours. Its absorption is not affected by food, so it can be taken alone or with food. In vivo, risperidone by CYP2D6 metabolism into 9-hydroxy-risperidone, the latter and risperidone have similar pharmacological effects, risperidone and 9-hydroxy-risperidone together constitute the product antipsychotic active ingredients, risperidone in vivo another metabolic pathway for N-demeronation. The elimination half-life of risperidone is about 3 hours, and the elimination half-life of 9-hydroxy-risperidone and other active metabolites is 24 hours. Most patients achieve a steady state of risperidone within 1 day and a steady state of 9-hydroxy-risperidone after 4 to 5 days. Within the therapeutic dose range, the blood concentration of risperidone is proportional to the administered dose. This product can be rapidly distributed in the body, the distribution volume is 1 ~ 2L/kg, in plasma, risperidone and albumin and the combination of 1 acid glycoprotein, risperidone plasma protein binding rate of 88%,9-hydroxy-risperidone plasma protein binding rate of 77%. After 1 week of medication, 70% of the drug is excreted through urine, 14% of the drug is excreted through stool, 35-45% of the excreted part through urine are risperidone and 9-hydroxy-risperidone, and the rest are inactive metabolites. A single dose study shows that the plasma concentration of the active ingredient of this product is higher in elderly patients and patients with renal insufficiency, and the clearance rate of the active ingredient is reduced by 30% in elderly patients, in patients with renal insufficiency decreased by 60%. Plasma concentrations of risperidone are normal in patients with hepatic insufficiency, but the unbound fraction of risperidone in plasma is increased by an average of about 35%. The pharmacokinetics of risperidone, 9-hydroxy-risperidone, and other antipsychotic active metabolites in children are similar to those in adults.
[Storage] 15~30 DEG C to save, do not freeze.
[packaging] brown medicinal poly cool bottle with white high density polyethylene lid, 30ml/bottle [validity] 36 months
[Implementation Standard] [Approval Document No. Tough Eagle Portuguese Drama Mystery] [Production Enterprise]]
Company: Yueyang Xinhuada Pharmaceutical Co., Ltd. Production Address: Wujiangqiao, Yueyang Economic And Technological Development Zone Postal Code: 414000
Telephone number: 0730-8258800 Fax number: 0730-8257700 Website: www.yyxhd.com
[[Holder of Listing Permit] Yueyang Xinhuada Pharmaceutical Co., Ltd.
Related Product recommend
online message
Filling in your phone and email information will help us get in touch with you in a timely manner and solve your problem as soon as possible.
 Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province
Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province  +86 730 8258800
+86 730 8258800  yyxhd_yt@vip.163.com
yyxhd_yt@vip.163.com

