
Aripiprazole Oral Solution
Key words:
Tablets, capsules, granules, topical solutions, oral solutions, suppositories, ointments
Classification:
Product Description
Aripiprazole Oral Solution Instructions
Please read the instructions carefully and make the warning under the guidance of the doctor.
Increased mortality in elderly patients with dementia-related psychosis
Elderly patients with dementia-related psychosis are at increased risk of death when treated with antipsychotic medications. This product is not approved for the treatment of dementia-related psychosis.
[Name of Drug]]
Generic Name: Aripiprazole Oral Solution
英文名称:Aripiprazole Oral Solution
汉语拼音:Alipaizuo Koufurongye
[ingredients]]
Active Ingredient: Aripiprazole
Chemical name: 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl] butoxy]-3,4-dihydroquinolone
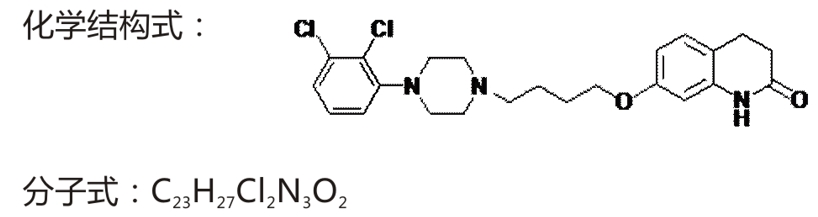
Molecular weight: 448.39
Excipients: lactic acid, glycerin, propylene glycol, disodium edetate, sodium hydroxide, methyl paraben, propyl paraben, fructose, sucrose, orange flavor
character]
This product is colorless to slightly yellow clear viscous liquid.
indications]
It is used for schizophrenia in adolescents and adults aged 13 to 17 years.
[specification]]
150ml:150mg
Usage and dosage]
Oral, once daily, not affected by food.
Adult
The recommend starting dose and target dose of this product is 10 or 15m/day, which is not affected by food. The clinical effective dose range is 10~25m 1/day. After 2 weeks of medication, the dose can be gradually increased to a maximum of 25m according to the individual's efficacy and tolerance. Thereafter, this dose can be maintained unchanged.
Maintenance treatment:The efficacy of maintenance treatment with aripiprazole should be evaluated periodically to determine whether to continue maintenance treatment.
Teenagers
The recommend target dose of this product is 10ml/day. The initial daily dose was 2ml, escalated to 5ml after two days, and to a target dose of 10ml after a further two days. Thereafter, the dose was increased by a dose amplitude of 5ml, but the maximum daily dose did not exceed 25ml.
When the dosage is 25mg or less, this product and aripiprazole oral solid preparation can be converted according to the relationship of 1ml:1mg; When the dosage is greater than 25mg, 25ml of this product is equivalent to 30mg of aripiprazole oral solid preparation (see [Pharmacokinetics] for details).
When switching from other antipsychotics to this product
Data on switching from other antipsychotics to aripiprazole or aripiprazole in combination with other antipsychotics in patients with schizophrenia have not been systematically collected. Although some patients may be able to accept immediate discontinuation of previous antipsychotic medications, gradual discontinuation may be more appropriate. In all cases, the duration of antipsychotic overlap should be minimized.
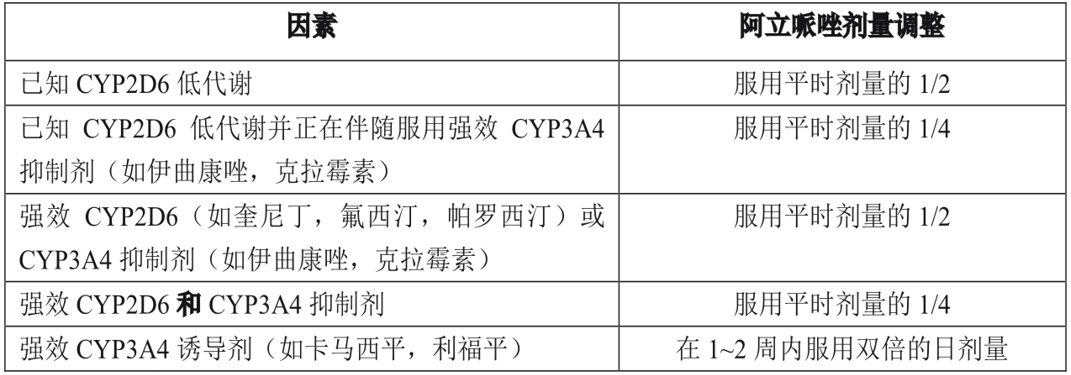
Dose Adjustment
Dose adjustment is recommended in patients with known CYP2D6 hypometabolism and who are taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or potent CYP3A4 inducers (see Table 1). When the concomitant use of the drug is withdrawn from concomitant therapy, the dose of aripiprazole should be adjusted to the original level. When simultaneously administered CYP3A4 inducers are discontinued, the dose of aripiprazole should be reduced to the original dose within 1 to 2 weeks. At the beginning of simultaneous administration of strong, medium or weak CYP3A4 and CYP2D6 inhibitors (for example, 1 strong CYP3A4 inhibitor and 1 medium CYP2D6 inhibitor or 1 medium CYP3A4 inhibitor and 1 medium CYP2D6 inhibitor), the dose of aripiprazole should be reduced to 1/4 of the usual dose, and then the dose should be adjusted to obtain good clinical efficacy.
Table 1. Dose adjustment in patients with known CYP2D6 hypometabolism and concomitant CYP2D6 inhibitors, CYP3A4 inhibitors, and/or CYP3A4 inducers
adverse reaction]
1, Safety data based on clinical trials in adults
The safety of aripiprazole was evaluated in a multiple-dose clinical trial involving 13543 adult patients; oral aripiprazole exposure was approximately 7619 case-years. In total, 3390 patients were treated with oral aripiprazole for at least 180 days and 1933 for at least 1 year.
The only common adverse reaction associated with the use of aripiprazole in patients with schizophrenia (5% or more, and at least twice as common in the aripiprazole group as in placebo) was akaidosis (8% in the aripiprazole group; 4% in placebo).
Table 2 lists the incidence of adverse reactions during acute treatment (up to 6 weeks for schizophrenia and up to 3 weeks for manic episodes of bipolar disorder), including only those with an incidence of> 2% or greater than placebo in patients treated with aripiprazole (dose> 2mg/day).
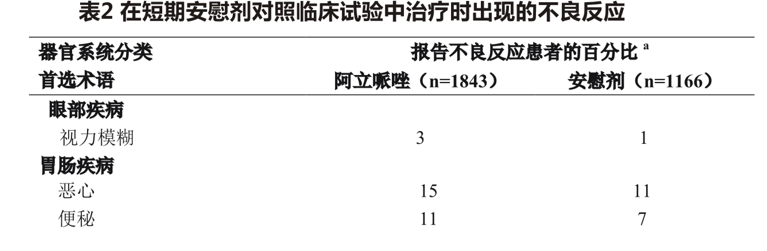
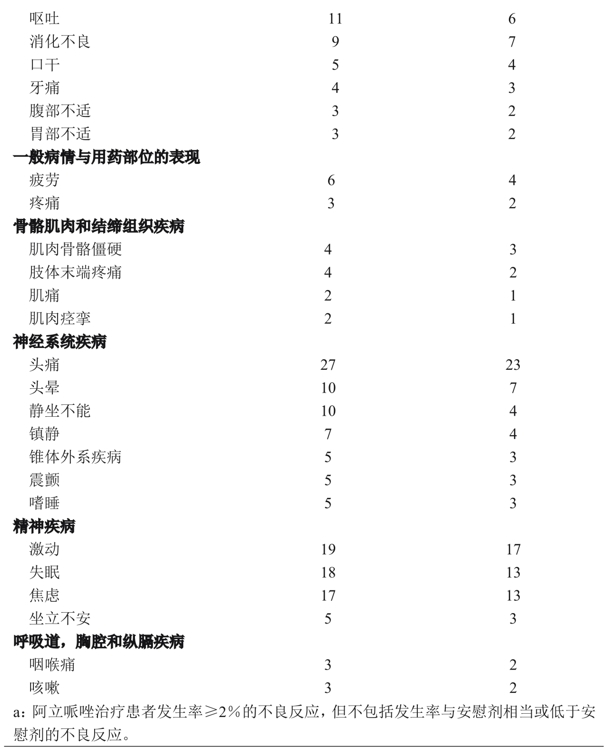
Examination of subgroups of the population found no clear evidence of differences in the incidence of adverse events by age, sex, or ethnicity.
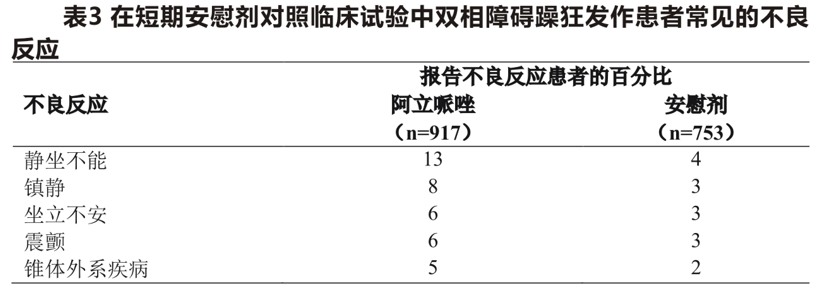
Based on the results of a 3-week placebo-controlled bipolar mania trial, the oral aripiprazole dose was 15 or 30mg/day. Common adverse reactions associated with the use of aripiprazole in patients with manic episodes of bipolar disorder (incidence> 5%, at least twice as high in the aripiprazole group as in the placebo group) are shown in Table 3.
Pediatric patients (13-17 years)
The following results are based on a 6-week, placebo-controlled trial of oral aripiprazole in doses ranging from 2 to 30mg/day. Discontinuation-related adverse reactions
The incidence of discontinuation due to adverse events in pediatric patients (13 to 17 years of age) treated with aripiprazole and placebo was 5% and 2%, respectively. Common observed adverse reactions
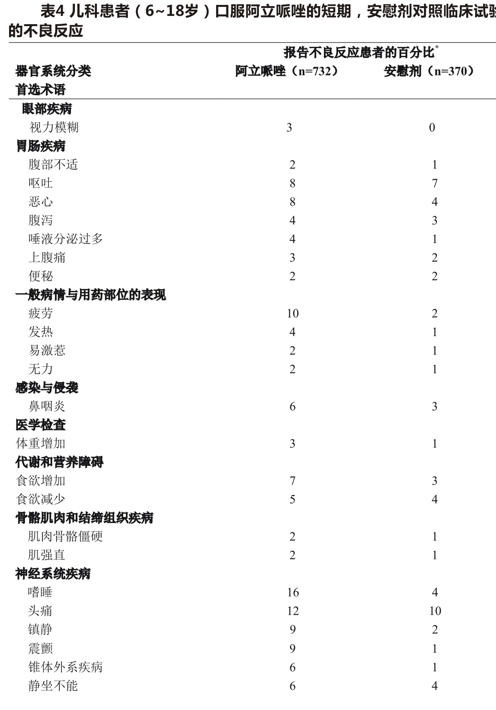
Common observed adverse effects of aripiprazole in adolescents with schizophrenia (incidence> 5% and at least twice the incidence of placebo treatment) are extrapyramidal disorders, sleepiness, and tremor. Table 4 lists the combined incidence of adverse reactions during the acute treatment period (up to 6 weeks for schizophrenia, up to 4 weeks for bipolar mania, up to 8 weeks for autism, and up to 10 weeks for Tourette syndrome), rounded to the nearest percentage, including only aripiprazole treatment (dose> 2mg/day) in patients with an incidence of> 2% and aripiprazole treatment higher than placebo treatment.

Discontinuation-related adverse reactions:
Common adverse reactions (> 5% and at least twice as common as aripiprazole compared with placebo) in pediatric patients with schizophrenia (13 to 17 years of age) are extrapyramidal disorders, sleep, and tremor.
Dose-related Adverse Reactions
In four placebo-controlled clinical trials of oral aripiprazole at different fixed doses (2, 5, 10, 15, 20, and 30mg/day) in adult patients with schizophrenia, the dose-response relationship for the incidence of treatment-emergent adverse events was evaluated. Stratified analysis indicated that the only adverse reaction likely to have a dose-effect relationship and most pronounced only at 30mg was sleep (including sedation) (placebo: 7.1%;10mg:8.5%;15mg:8.7%;20mg:7.5%;30mg:12.6%).
In studies of pediatric patients with schizophrenia (13-17 years), three common adverse reactions may have a dose-response relationship: extrapyramidal disease (placebo incidence: 5.0; 10mg:13.0; 30mg:21.6); sleep (placebo incidence: 6.0; 10mg:11.0; 30mg:21.6); tremor (placebo incidence: 2.0; 10mg:2.0; 30mg:11.8 per cent).
extrapyramidal syndrome
In short-term placebo-controlled trials in adults with schizophrenia, the incidence of extrapyramidal syndrome (EPS)(other than akadia-related events) reported by patients in the aripiprazole and placebo groups was 13% and 12%, respectively; the incidence of akadia-related adverse events was 8% and 4%, respectively. In a short-term placebo-controlled trial in pediatric schizophrenia (13 to 17 years of age), the incidence of reported extrapyramidal syndrome (EPS)(other than akadia-related events) was 25% in the arizole-treated group and 7% in the placebo group, respectively; the incidence of akadia-related adverse events was 9% and 6% in the two groups, respectively.
The test was based on the Simpson-Angus (Simpson Angus) evaluation quantitative table (evaluation of EPS) Barnes (Barnes) akathisia scale (evaluation of akathisia) and involuntary movement evaluation scale (evaluation of dyskinesia) objective collection of data. In the adult schizophrenia trial, there was no difference in scores between the two groups except for the Barnes akathisia score (aripiprazole: 0.08, placebo:-0.05). In the Pediatric Schizophrenia (13-17 years) trial, objectively collected data showed no difference in scores between the aripiprazole and placebo groups, with the exception of the Simpson-Angus (Simpson Angus) rating scale score (arly, 0.24; placebo,-0.29). Similarly, in a long-term (26-week) placebo-controlled trial of schizophrenia, these scores did not differ between the two groups.
Dystonia
Dystonia, characterized by persistent abnormal muscle contractions, may occur in susceptible patients within the first few days of treatment. It is manifested by neck muscle spasms, sometimes leading to throat tightness, difficulty swallowing, dyspnea, and/or tongue spitting. These manifestations can occur at low doses, but usually occur at higher doses and are more severe. The risk of developing acute dystonia is increased in men and young patients.
Other results observed in clinical trials
Adverse effects in long-term, double-blind, placebo-controlled trials
The adverse reactions reported in a 26-week double-blind clinical trial comparing aripiprazole and placebo in schizophrenia patients were basically the same as those reported in a short-term placebo-controlled clinical trial, except that the incidence of tremor was higher [aripiprazole 8%(12/153) and placebo 2%(3/153)], and the severity of most tremor cases was mild (8/12 mild, 4/12 moderate), occurred in the early stage of treatment (9/12 ≤ 49 days), and the duration was limited (7/12 ≤ 10 days), rarely leading to aripiprazole discontinuation (<1%). In addition, in the long-term (52 weeks) positive drug
In the control study, the incidence of tremor in the aripiprazole group was 5% (859). A similar picture can be observed in studies of manic episodes of long-term bipolar disorder. 2. Other adverse reactions observed during the pre-marketing evaluation of aripiprazole The following list does not include the following adverse reactions: 1) Adverse reactions listed in the previous table or other parts of the instructions: 2) Adverse reactions that are far from the causal relationship with the drug; 3) Adverse reactions that are very common and therefore have no information value: 4) Adverse reactions that are considered to have no significant clinical significance; 5) The incidence of adverse reactions was lower than that of placebo group subjects. Reactions were classified by body system according to the following definitions: common adverse reaction rate> 1/100; occasional adverse reaction rate 1/100~1/1000; rare adverse reaction rate ≤ 1/1000:
Adults-oral
Blood and lymphatic system diseases
Rare Thrombocytopenia
Heart disease:
One bradycardia and palpitation were occasionally seen;
Rare-atrial flutter, cardiopulmonary arrest, atrioventricular block, atrial fibrillation, angina pectoris, myocardial ischemia, myocardial infarction, cardiopulmonary failure;
Eye diseases:An occasional photophobia rare diplopia
Gastrointestinal diseases:One gastroesophageal reflux was occasionally seen;
The general condition and the performance of the medication site:
Common a weakness;
Occasional-peripheral edema, chest pain
Rare-facial edema;
liver and gallbladder diseases:
A rare hepatitis, jaundice;
Immune system diseases:
rare a hypersensitivity reaction;
Trauma, poisoning and operational complications:
I see a fall;
Rare-heat stroke;
Medical Examination:
Common-weight loss;
I occasionally saw an increase in liver enzyme, blood sugar, blood lactate dehydrogenase and y-glutamyltransferase.
High
Rarely, blood prolactin increased, blood urea increased, serum creatinine increased, blood bilirubin increased, ECG QT interval prolonged, glycosylated hemoglobin increased;
Metabolic and nutritional disorders:
Common-Anorexia:
Rare Hypokalemia, Hyponatremia, Hypoglycemia
Musculoskeletal and connective tissue disorders:
I see a muscle weakness, muscle tension;
Rare-rhabdomyolysis, decreased mobility;
Diseases of the nervous system:
Ginsen 1 Pa, memory impairment, cogwheel-like rigidity, hypokinesia, and bradykinesia are occasionally seen;
Rare-motor inability, myoclonus, coordination abnormalities, speech disorders, epilepsy grand mal;<1/10000 patients with a dancing hand foot movement; mental disorders:
occasionally see an aggressive, loss of libido, delirium;
Rare Increased libido, anorgasmia, smoking, homicidal ideation, catatonia, sleeping disorder
kidney and urinary system diseases:
Rare one urine pig stay, enuresis;
diseases of the reproductive system and breast:
I occasionally see an erectile dysfunction;
Uncommon Gynecomastia in Men, Irregular Menorrhagia, Amenorrhea, Breast Pain, Persistent Penile Erection
Respiratory, thoracic and mediastinal disorders: occasional nasal obstruction, dyspnea;
Skin and subcutaneous tissue disorders:
Occasionally see a rash, sweating, skin itching, photosensitive reaction, hair loss;
rare-urticaria;
Vascular disease:
I see a low blood pressure, high blood pressure,
Pediatric patients-oral
Most of the adverse events observed in the pooled database of 1686 pediatric patients (6-18 years) were also detected in adults. Other adverse reactions observed in pediatric patients are listed below. Eye diseases:
Ocular-oculomomo crisis
Gastrointestinal diseases:
Occasionally-dry tongue, tongue spasm
Check:
Common-Increased blood insulin
Diseases of the nervous system:
I see you-talking in my sleep
kidney and urinary system diseases:
Common-Enuresis
Skin and subcutaneous tissue disorders:
Ocdental-hirsutism
3. Other adverse reactions observed during the post-marketing evaluation of aripiprazole
The following adverse reactions have been demonstrated in post-approval use of aripiprazole. Since these reactions are spontaneous reports from an indeterminate population, it is not always possible to establish a causal relationship between adverse reactions and drug exposure: allergic reactions (e. g., anaphylaxis, angioedema, laryngeal spasm, pruritus/measles, or oropharyngeal spasm), pathological gambling, snoring, blood fluctuations, oculomotor crisis, and drug reactions with eosinophilia and systemic symptoms (DRESS) occurred.
taboo]
It is contraindicated in patients with known allergy to this product.
[Note]]
1. Increased mortality in elderly patients with dementia-related psychosis Increased mortality Increased risk of death with antipsychotic drugs in elderly patients with dementia-related psychosis. This product (aripiprazole) is not approved for the treatment of patients with dementia-related psychiatric disorders.
Safety experience for elderly psychiatric patients with concomitant Alzheimer's disease:
In three placebo-controlled trials of 10-week aripiprazole treatment of elderly psychiatric patients with Alzheimer's disease (n = 938, mean age: 82.4 years; age range: 56-99 years), the incidence of adverse events on treatment was> 3% and the incidence of aripiprazole was at least twice that of placebo, including drowsiness (placebo 2%, aripiprazole 5%), sleep (placebo 3%, aripiprazole 8%) and urinary incontinence (placebo 1%, ariprazole 5%), hypersalivation (placebo 0%, aripiprazole 4%), dizziness (placebo 1%, aripiprazole 4%).
The safety and efficacy of aripiprazole in the treatment of patients with dementia-related psychosis have not been established. Care should be taken if physicians choose to treat these patients with aripiprazole, especially those with dysphagia.
Difficulty or excessive sleepiness may result in accidental injury or aspiration.
2, cerebrovascular adverse events, including brain witch.
In placebo-controlled clinical trials of dementia-related psychosis (2 unfixed doses and 1 fixed dose), treatment with aripiprazole was associated with an increased incidence of cerebrovascular adverse events (eg, stroke, transient ischemic attack), including death, in elderly patients (mean age, 84 years; age range, 78-88 years). The results of the fixed dose test showed that there was a dose-response relationship between cerebrovascular adverse events and drugs, which was statistically significant. Aripiprazole cannot be used to treat dementia-related psychosis
Sick patients.
Neuroleptic Malignant Syndrome (NMS)
Neuroleptic malignant syndrome (NMS) is a potentially fatal syndrome associated with the use of antipsychotic drugs, including aripiprazole. According to the aripiprazole pre-marketing global clinical database, NMS is rare. Clinical manifestations of NMS are hyperthermia, myotonia, altered mental status, and signs of autonomic instability (irregular fluctuations in pulse or blood pressure, tachycardia, sweating, and arrhythmias). Other symptoms may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnosis of NMS is complex. It is important to exclude clinical manifestations that are accompanied by serious medical conditions (eg, pneumonia, systemic infections) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Another important consideration in differential diagnosis includes central anticholinergic toxicity, heat stroke, drug-induced fever, and primary central nervous system disorders.
Management of NMS: 1) Immediately stop taking antipsychotics and other current non-essential treatment drugs; 2) Intensifying symptomatic treatment and medical monitoring; 3) Treat the accompanying serious medical problems (with feasible specific therapy). There is currently no recognized specific drug treatment for uncomplicated NMS.
If a patient still requires antipsychotic therapy after recovery from NMS, the possibility of reinitiating NMS with pharmacotherapy should be carefully considered. Recurrence of NMS has been reported, therefore, close monitoring should be strengthened.
4. Tardive Dyskinesia
In patients treated with antipsychotics, an irreversible syndrome of involuntary dyskinesia may occur. The incidence of this syndrome is highest in elderly patients (especially in elderly women), but it is impossible to predict which patients will develop the syndrome at the beginning of antipsychotic treatment based on epidemiology alone. It is unclear whether there is a difference in the likelihood that antipsychotic drugs cause tardive dyskinesia. The risk of developing tardive dyskinesia and the likelihood of its appearance being irreversible increase with the duration of treatment and the increase in the total cumulative dose of antipsychotic drugs used. However, the syndrome may occur after relatively short-term low-dose treatment, but is less common.
If tardive dyskinesia is partially or completely relieved after stopping antipsychotic therapy. However, antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of this syndrome, which may mask the progression of the disease. The long-term effects of symptom suppression on the syndrome are unclear. Based on the above considerations, aripiprazole should be used in a way that minimizes the occurrence of tardive dyskinesia. Long-term antipsychotic therapy should be reserved for patients with chronic diseases:(1) treatment with antipsychotics is known to be effective; and (2) treatment with less potential harm is not available or appropriate. Long-term treatment should seek the lowest therapeutic dose and shortest treatment time that can achieve satisfactory results, and the need for continuous treatment should be periodically re-evaluated.
If patients receiving aripiprazole develop signs and symptoms of tardive dyskinesia, discontinuation should be considered. These symptoms may worsen temporarily even after treatment is discontinued. Although some patients develop this syndrome, they still need to be treated with aripiprazole.
5. Metabolic changes
High blood sugar/diabetes
Severe hyperglycemia accompanied by ketoacidosis or hyperosmolar coma or death has been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia induced by aripiprazole. Epidemiological studies suggest an increased risk of hyperglycemia-related adverse effects in patients treated with atypical antipsychotics. Patients with a definite diagnosis of diabetes at the time of initiation of atypical antipsychotic therapy should be regularly monitored for worsening of glycemic control. Patients with risk factors for diabetes (eg, obesity, family history of diabetes) should have fasting blood glucose tested regularly before and during treatment. Symptoms of hyperglycemia, including polydipsia, polyuria, polydipsia, and weakness, should be monitored. In some patients, hyperglycemia disappears on its own when atypical antipsychotic therapy is discontinued; however, in some patients, hypoglycemic therapy is continued despite discontinuation of the suspected drug.
Adult
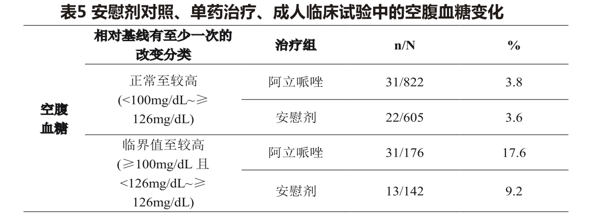
In an analysis of 13 placebo-controlled, monotherapy, adult (primarily patients with schizophrenia or bipolar disorder) clinical trials, mean changes in fasting glucose were not significantly different between aripiprazole-treated patients (4.4mg/dL; median exposure days, 25 days: N = 1057) and placebo-treated patients (2.5mg/dL; median exposure days, 22 days; N = 799). Table 5 shows the proportion of patients in the aripiprazole treatment group with normal and borderline fasting glucose baseline with higher fasting glucose levels (median exposure of 25 days) compared with the placebo group (median exposure of 22 days).
Pediatric patients and adolescents
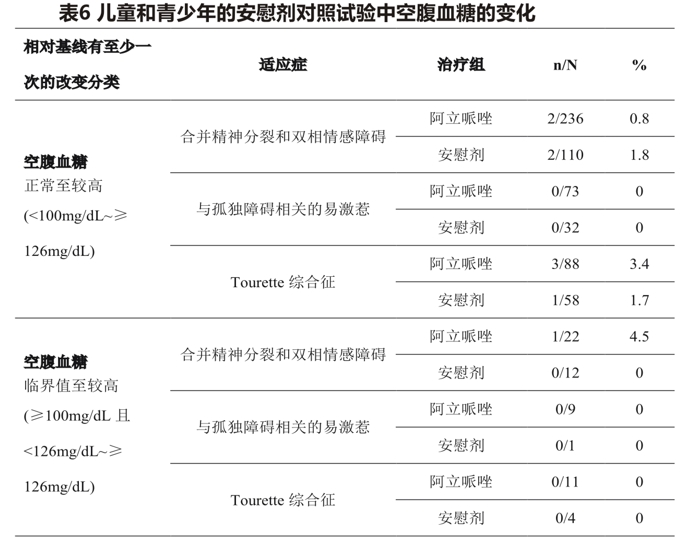
In two analyses of placebo-controlled trials in adolescent patients with schizophrenia (13 to 17 years of age) and pediatric bipolar disorder (10 to 17 years of age), the mean change in fasting glucose was not significantly different in patients treated with aritin (4.8mg/dL; median exposure days, 43 days; N = 259) compared with patients treated with placebo (1.7mg/dL; median exposure days, 42 days; N = 123). In an analysis of 2 placebo-controlled trials of irritable patients (6-17 years) associated with autistic disorder, the mean change in fasting plasma glucose was not significantly different in aripiprazole-treated patients (-0.2mg/dL;N = 83) compared with placebo-treated patients (-0.6mg/dL;N = 33) for a median of 56 days of exposure.
In an analysis of 2 placebo-controlled trials in pediatric and adolescent patients (6-18 years) with the Tourette syndrome, the mean change in fasting glucose was not significant in aripiprazole-treated patients (0.79mg/dL; N = 90) compared with placebo-treated patients (-1.66mg/dL; N = 58) with a median exposure of 57 days.
The difference. Table 6 shows the proportion of patients with changes in fasting blood glucose levels, which pooled adolescent schizophrenia and pediatric bipolar disorder patients (median exposure days 42-43 days), and 2 placebo-controlled trials (median exposure days 56 days) in irritable pediatric patients (6-17 years) associated with autistic disorder, two placebo-controlled trials in patients (6-18 years) with Tourette syndrome (median exposure days, 57).
 During the 12-week pooled trials of adolescent schizophrenia and pediatric bipolar disorder, the mean change in fasting glucose was not significantly different between aripiprazole-treated patients and placebo-treated patients [2.4mg/dL(n = 81) and 0.1mg/dL(n = 15), respectively].
During the 12-week pooled trials of adolescent schizophrenia and pediatric bipolar disorder, the mean change in fasting glucose was not significantly different between aripiprazole-treated patients and placebo-treated patients [2.4mg/dL(n = 81) and 0.1mg/dL(n = 15), respectively].
Dyslipidemia
Undesirable lipid changes were already seen in patients treated with atypical antipsychotics There was no significant difference between the aripiprazole and placebo groups in the proportion of patients who changed from baseline to clinically meaningful levels of fasting/non-fasting total cholesterol, fasting triglycerides, fasting LDL, and fasting/non-fasting HDL.
Adult
Table 7 summarizes total cholesterol (17 trials; median exposure days, 21-25 days), fasting triglycerides (8 trials; median exposure days, 42 days), fasting LDL cholesterol (8 trials; median exposure days, 39-45 days), the median duration of treatment exposure was 24 days) and the proportion of adult patients with changes in HDL cholesterol (pooled 9 clinical trials; median exposure days ranged from 40 to 42 days).
Table 7 Changes in lipid parameters in placebo-controlled, monotherapy, adult clinical trials
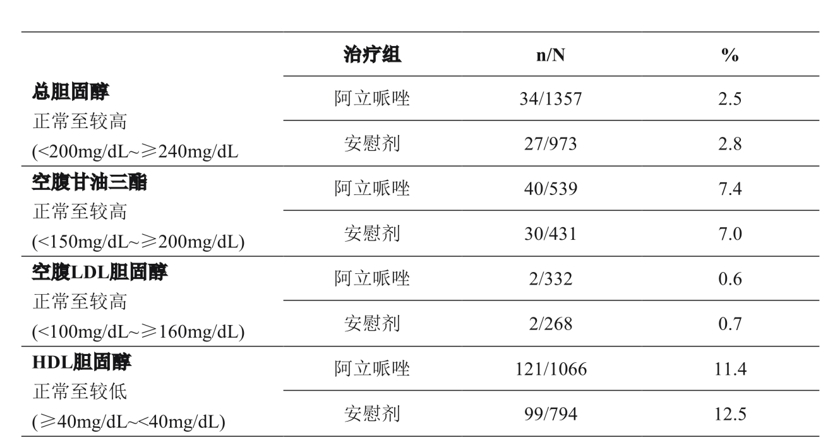
In the adult monotherapy clinical trial, the changes in total cholesterol (fasting/non-fasting), fasting triglycerides, and fasting LDL cholesterol from normal to higher levels at 12 weeks and 24 weeks were similar for patients treated with apirate and placebo.
Pediatric patients and adolescents Table 8 shows the proportion of patients with changes in total and HDL cholesterol (pooled 2 placebo-controlled trials; median exposure days 42-43 days) and fasting triglycerides (pooled 2 placebo-controlled trials; median exposure days 42-44 days) in adolescents with schizophrenia (13-17 years) and pediatric bipolar disorder (10-17 years).
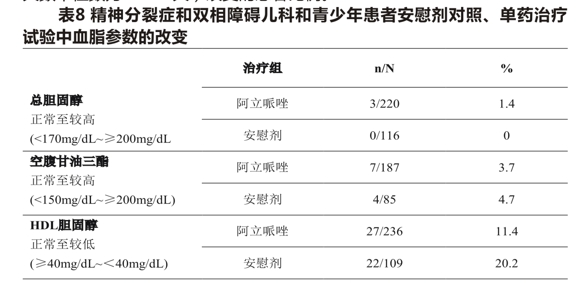
Comparison of the proportion of patients with a change from normal to higher levels of total cholesterol (fasting/non-fasting), fasting triglycerides, and fasting LDL cholesterol at 12 and 24 weeks in a monotherapy clinical trial in adolescent schizophrenia and pediatric bipolar disorder was similar in the aripiprazole and placebo groups: at 12 weeks, total cholesterol (fasting/non-fasting) 0/57(0%)vs. 0/15(0%); fasting triglycerides, 2/72(2.8) vs. 1/14(7.1); at 24 weeks total cholesterol (fasting/non-fasting) 0/36(0%)vs. 0/12(0%); fasting triglycerides, 1/47(2.1 percent) vs. 1/10(10.0 percent).
Table 9 shows the proportion of patients with changes in total cholesterol (fasting/non-fasting), fasting triglycerides (median exposure days 56 days), and HDL cholesterol (median exposure days 55-56 days) in 2 placebo-controlled clinical trials in pediatric (6-17 years) irritable patients associated with autism disorder.
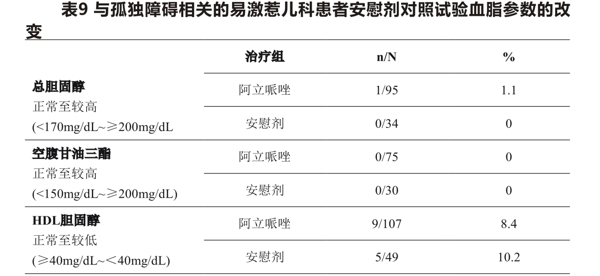
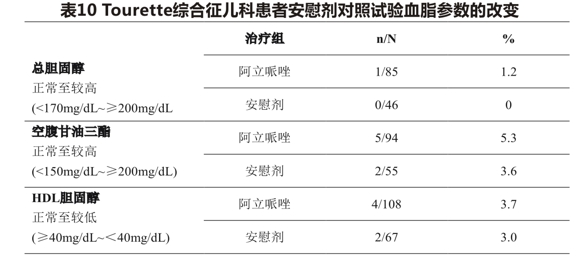
Table 10 shows the proportion of patients with changes in total cholesterol (fasting/non-fasting), fasting triglycerides (median exposure days 57 days), and HDL cholesterol (median exposure days 57 days) in 2 placebo-controlled clinical trials in pediatric (6-18 years) patients with Tourette syndrome.
Weight gain
There have been reports of weight gain from the use of atypical antipsychotics. Clinical monitoring of body weight was recommend in patients treated with atypical antipsychotics.
Adult
In the clinical trial of aripiprazole plus antidepressant therapy, patients were treated with antidepressants for 8 weeks first, followed by aripiprazole or placebo plus antidepressant therapy for 6 weeks. The mean change in body weight was +1.7kg(N = 347) for patients taking the aripiprazole add-on therapy and +0.4kg(N = 330) for patients in the placebo add-on therapy. Table 11 shows the percentage of adult patients with weight gain> 7% of baseline weight for each indication.
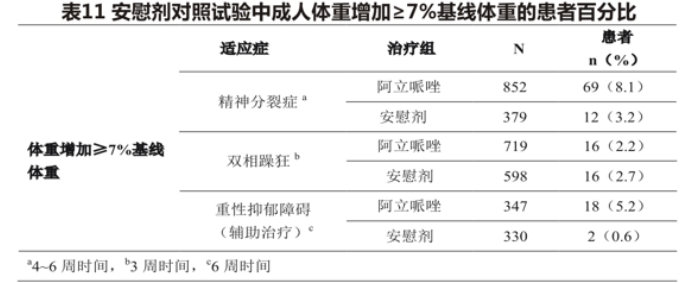
Pediatric and adolescent patients
In an analysis of two placebo-controlled trials (median days of exposure 42-43 days) in adolescent schizophrenic patients (13-17 years) and pediatric bipolar disorder (10-17 years), the mean change in body weight was +1.6kg(N = 381) in aripiprazole-treated patients compared with +0.3kg(N = 187) in placebo-treated patients. The mean change in body weight from baseline in aripiprazole-treated patients was +5.8kg(n = 62) versus +1.4kg(n = 13) in the placebo group over a 24-week period. In two short-term, placebo-controlled trials (median days of exposure 56 days) in patients (6-17 years) with autism-related irritability, the mean change in body weight was +1.6kg(n = 209) compared with +0.4kg(n = 98) with placebo. In two short-term, placebo-controlled trials in patients (6-18 years) with the Tourette syndrome (median exposure days, 57 days), the mean change in body weight was +1.5kg(n = 105) on aripiprazole versus +0.4kg(n = 66) on placebo. Table 12 shows the percentage of pediatric and adolescent patients with weight gain> 7% of body weight.
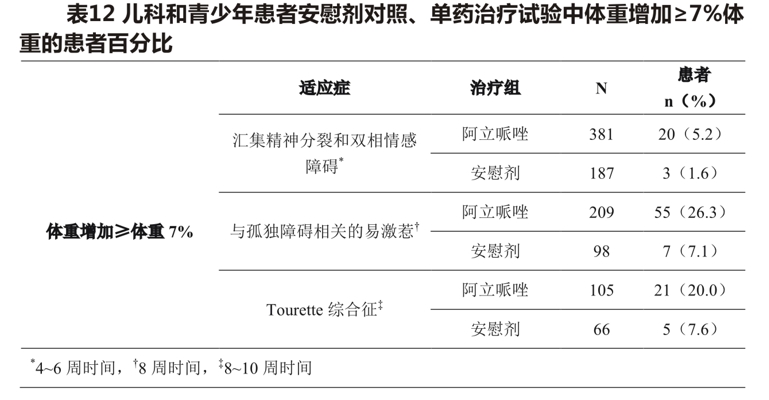
Adolescent patients with schizophrenia (13 to 17 years of age) and pediatric patients with bipolar disorder (10 to 17 years of age) from two placebo-controlled trials were enrolled in one open-label trial, and 73.2 percent of patients (238/325) completed 26 weeks of aripiprazole. After 26 weeks, 32.8 percent of patients gained> 7 percent of their body weight, with no adjustment for normal gain. To adjust for normal growth, z-scores (measured by standard deviation [SD]) were derived for pediatric and adolescent patients whose normal growth was standardized by age-and sex-matched population criteria. Change in z-score <0.5SD was considered clinically insignificant. after 26 weeks, the mean change in z-score was 0.09SD.
From 2 short-term, placebo-controlled trials of irritable patients (6 to 17 years of age) associated with autistic disorder and new patients enrolled in 1 open-label trial, 60.3% of patients (199/330) completed 1 year of aripiprazole treatment. the mean change in weight z-score for patients treated for> 9 months was 0.26SDs.
When treating pediatric patients for any indication, weight gain should be monitored and assessed for expected normal growth.
Pathological Gambling and Other Impulse Control Disorders
Post-marketing reports indicate that patients may experience increased impulsivity, especially gambling, and inability to control these impulsivity when taking aripiprazole. Other less frequent compulsions include increased sexual impulses, compulsive consumption, overeating or compulsive eating, and other impulsive or compulsive behaviors. Because patients may not perceive these behaviors as abnormal, prescribing physicians specifically ask patients or their caregivers about emerging or increasing gambling impulses, sexual impulses, compulsive consumption, binge or compulsive eating, and other impulsive behaviors. It should be noted that impulse control symptoms may be related to the underlying disease. In some cases, not all cases, the impulsive behavior is gone after the dose is reduced or stopped. If not recognized, compulsive behavior may cause harm to the patient or others. If patients develop these impulses while taking aripiprazole, consideration should be given to reducing the dose or discontinuing the drug.
7, low position
Blood pressure Aripiprazole has an antagonistic effect on α,-adrenergic receptors and may cause orthostatic hypotension. In short-term placebo-controlled trials of aripiprazole in adults with schizophrenia (n = 2467), the incidence of events associated with orthostatic hypotension included orthostatic hypotension (placebo 0.3%, aripiprazole 1%), orthostatic dizziness (placebo 0.3%, aripiprazole 0.5%), and syncope (placebo 0.4%, aripiprazole 0.5%).
There was no meaningful difference in the incidence of significant orthostatic changes in blood pressure (defined as a> 20mm Hg decrease in systolic blood pressure and> 25 increases in heart rate after standing compared with lying flat) compared with oral placebo (aripiprazole 4% vs. placebo 2%).
With caution, aripiprazole should be used in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure, or conduction abnormalities), cerebrovascular disease, or conditions that can cause hypotension (dehydration, hypovolemia, and treatment with antihypertensive drugs)
8, fall.
Antipsychotic drugs, including aripiprazole, may cause somnolence, orthostatic hypotension, motor and sensory instability, and these events may lead to falls, which may lead to fractures or other fall-related injuries. Patients whose own diseases, conditions, or medications may exacerbate the above effects should have a complete fall risk assessment when starting their antipsychotic treatment, if the patient is undergoing long-term treatment.
Antipsychotic therapy requires repeated evaluation.
9, Leukopenia, neutropenia, agranulocytosis
Events of leukopenia/neutropenia temporarily associated with antipsychotic drugs, including aripiprazole, have been reported in clinical trials and/or post-marketing experience. Agranulocytosis has also been reported
Possible risk factors for leukopenia/neutropenia include a history of low white blood cell count (WBC)/absolute neutrophil count (ANC) and drug-induced leukopenia/neutropenia. Such patients should frequently monitor their whole cell count (CBC) in the first few months of treatment, and in the absence of other precipitating factors, once clinically significant WBC occurs
It was considered to stop using aripiprazole. Patients with clinically significant neutropenia should be carefully monitored for symptoms or signs of fever or other infection and should be treated promptly if these symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm) should stop aripiprazole until WBC returns.
10, Epilepsy/Convulsions
In short-term, placebo-controlled clinical trials, excluding patients with a history of epilepsy, oral aripiprazole was reported to have epilepsy/convulsions in 0.1%(2467) of adult patients.
As with other antipsychotics, aripiprazole should be used with caution in patients with a history of epilepsy or in patients with a low epileptic threshold (eg, Alzheimer's disease dementia). Low epilepsy values are more common in people ≥ 65 years of age.
11, Potential Cognitive and Motor Impairment
Like other antipsychotics, aripiprazole may affect judgment, thinking, or motor skills. For example, in short-term, placebo-controlled trials, somnolence (including sedation) has been reported (aripiprazole incidence, placebo incidence) in adult patients treated with aripiprazole (n = 2467)(11%, 6%) and in pediatric patients aged 6 to 17 years (n = 611)(24%,6%),0.3%(8/2467) of adult patients and 3%(15/611) of pediatric patients (6-17 years old) discontinued the drug due to drowsiness (including sedation), but the aripiprazole-injected adult patients did not.
Although the increase in the incidence of these events is relatively low compared to placebo, patients who operate dangerous machinery, including driving vehicles, should be cautioned unless there is reason to determine that the use of aripiprazole will not have an adverse effect on the use of these events. Although the increase in the incidence of these events is relatively low compared to placebo, patients who operate dangerous machinery, including driving vehicles, should be cautioned unless there is reason to determine that the use of aripiprazole will not have an adverse effect.
12, Thermoregulation
Antipsychotic drugs can disrupt the body's ability to lower core body temperature. Adequate care is recommended when aripiprazole is prescribed for patients with conditions that may raise body temperature (such as strenuous exercise, overheating, concomitant use of anticholinergic drugs, or dehydration).
13 and suicide.
Suicidal tendencies are an inherent feature of mental illness, bipolar disorder, and major depressive disorder, and high-risk patients should be closely monitored during drug therapy. Aripiprazole should be prescribed with a minimum amount of therapeutic dose to reduce the risk of overdose.
14. Difficulty swallowing
Esophageal motor dysfunction and aspiration are associated with the use of antipsychotic drugs, including aripiprazole. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, especially in patients with advanced Alzheimer's dementia. Aripiprazole and other antipsychotics should be used with caution in patients at risk of aspiration pneumonia.
Medication for pregnant and lactating women]
Appropriate and well-controlled studies have not been conducted in pregnant women. It is not known whether the use of aripiprazole in pregnant women will cause fetal damage or affect reproductive capacity. For pregnant women, use this product only when the potential benefit to the fetus is higher than the potential danger. Patients should be informed that they are pregnant or intend to become pregnant when taking aripiprazole should inform the doctor. Neonates exposed to antipsychotic drugs (including aripiprazole) during the third trimester of pregnancy are at risk of developing extrapyramidal and/or withdrawal symptoms after birth. Adverse reactions that have been reported in newborns are agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorders. These complications vary in severity; some newborns recover within hours or days without special treatment; others require prolonged hospitalization. The effect of aripiprazole on labor pains and labor in pregnant women is unknown.
Aripiprazole can be secreted into the milk of lactating women. Consider the importance of this product to breastfeeding women before deciding whether to stop breastfeeding or stop taking the drug.
children medication]
The safety and efficacy of aripiprazole in pediatric patients with schizophrenia have been established in a 6-week placebo-controlled clinical trial involving 202 pediatric patients aged 13 to 17 years. Although the effectiveness of aripiprazole in the maintenance treatment of pediatric patients has not been systematically evaluated, the effectiveness of aripiprazole in the maintenance treatment of pediatric patients can be determined by extrapolating the data of adult patients by comparing the pharmacokinetic parameters of aripiprazole in adult patients and pediatric patients.
elderly medication]
Because a sufficient number of patients> 65 years of age were not enrolled in pre-marketing placebo-controlled trials for the treatment of manic episodes of schizophrenia and bipolar disorder, it is difficult to determine whether the response to treatment in older patients is consistent with that in younger patients. Elderly patients do not need to adjust the dose.
The safety and efficacy of aripiprazole in patients with dementia-related psychosis have not been established. This product is not approved for the treatment of dementia-related psychosis.
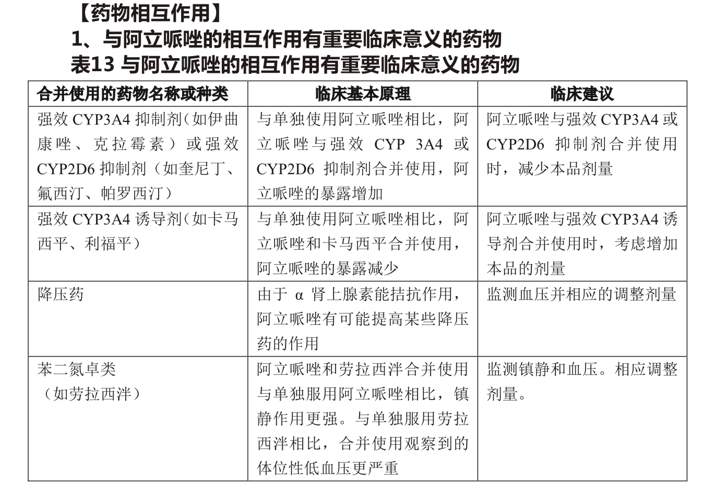
2. The interaction with aripiprazole has no important clinical significance
Based on pharmacokinetic studies, aripiprazole was administered concomitantly with famotidine, valproic acid, lithium, and lorazepam without dose adjustment.
In addition, CYP2D6 (such as dextromethorphan, fluoxetine, paroxetine, venlafaxine) CYP2C9 (such as warfarin),CYP2C19 (such as omeprazole, warfarin, escitalopram), or CYP3A4 (such as dextromethorphan) and aripiprazole need not be adjusted.
drug overdose]
Classification of adverse events using MedDRA terminology,
1, Clinical experience
At present, adverse reactions to intentional or accidental overdose of aripiprazole in clinical trials and post-marketing have been reported worldwide, including overdose of aripiprazole alone and when combined with other drugs. No deaths were reported from aripiprazole alone. Complete recovery occurred in patients with a maximum acute intake of 1260mg of aripiprazole (42 times the maximum daily recommend dose). ingestion of aripiprazole
Dosage (oral intakes up to 195 mg) was also reported in children (aged ≤ 12 years), but no deaths were reported. Common (at least 5%) adverse events reported with overdose of oral aripiprazole (alone or in combination) included vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in 21 patients with aripiprazole overdose (alone or in combination) included acidosis, aggressive behavior, elevated aspartate aminotransferase, atrial fibrillation, bradycardia, coma, confusion, convulsions, elevated creatine phosphokinase, low consciousness, hypertension, hypokalemia, hypotension, drowsiness, loss of consciousness, prolonged QRS complex duration, prolonged QT period, aspiration pneumonia, respiratory arrest, status epilepticus and tachycardia.
2. Excessive treatment
There is currently no specific method to rescue aripiprazole overdose. In case of overdose, ECG should be checked; if QT prolongation occurs, close cardiac monitoring should be performed. At the same time, supportive therapy should be used to maintain airway patency, oxygen and ventilation, symptomatic treatment. Close monitoring should be continued until the patient recovers.
Activated charcoal: If an aripiprazole overdose occurs, the early use of activated charcoal may help to prevent the absorption of aripiprazole to some extent. 1 hour after a single oral dose of 15mg aripiprazole, taking
50g of activated carbon reduced the mean AUC and C of aripiprazole by 50%. Hemodialysis: Although there is no information on the treatment of aripiprazole overdose on hemodialysis, hemodialysis may not have a significant effect on overdose due to the high plasma protein binding rate of aripiprazole
pharmacology and toxicology]
Pharmacological action
Aripiprazole has high affinity with D, D, 5-HT, 5-HT, receptors, and D, 5-HT. 5-HT, α, H, receptors and 5-HT reuptake sites have moderate affinities. Aripiprazole is a partial agonist of the D, receptor and 5-HT,A receptor, and also an antagonist of the 5-HT, receptor. As with other antischizophrenic drugs, the mechanism of action of aripiprazole is unclear. However, it is currently believed to be mediated by partial agonism of D, and 5-HT., receptors and antagonism of 5-HT receptors. Action with other receptors may produce some other clinical effects of aripiprazole, such as antagonism of the q, receptor, which may explain the phenomenon of orthostatic hypotension.
toxicological studies
Genotoxicity
In CHL cells in vitro chromosome aberration test, aripiprazole and its metabolite (2,3-DCPP) with or without metabolic activation caused chromosome breakage, metabolite 2,3-DCPP in the absence of metabolic activation increased the number of aberrations. The in vivo micronucleus test in mice was positive, but the results are thought to be produced by a mechanism unrelated to the human body. Aripiprazole was negative in the in vitro bacterial reverse mutation test, bacterial DNA repair test, mouse lymphoma cell test, and rat out-of-procedure DNA synthesis test.
reproductive toxicity
Female rats were orally given aripiprazole 2, 6 and 20 mg/kg/day from 2 weeks before mating to 7 days of pregnancy [in mg/m? It is equivalent to 0.6, 2 and 6 times of the maximum human recommend dose (MRHD) respectively]. Estrous cyclin disorder and corpus luteum increase were observed in all dose groups, but no damage to fertility was found. The loss before implantation increased in the 6 and 20 mg/kg dose groups and the fetal weight decreased in the 20 mg/kg group. The sexual rats were given aripiprazole 20, 40 and 60 mg/kg/day (in mg/m? equivalent to 6, 13 and 19 times of MRHD, respectively) by mouth from the 9th week before mating to the whole mating period. Sperm production disorders were found at the dose of 60 mg/kg, and prostatic atrophy was observed at the dose of 40 and 60 mg/kg, but no impairment of fertility was found.
Aripiprazole has been shown to have developmental toxicity in animal studies, including possible teratogenic effects in rats and domestic animals.
Pregnant rats were given aripiprazole 3, 10 and 30 mg/kg/day (in mg/m ", equivalent to 1, 3 and 10 times MRHD, respectively) orally during the organogenesis period, and the 30 mg/kg dose group had a mild prolongation of pregnancy, a slight delay in fetal development (reduced fetal weight), an increase in the incidence of undescended testicles by 10 and a delay in skeletal ossification in the 30 mg/kg dose group; there was no effect on the survival of embryo-fetus or young. The weight of the offspring delivered in the 10 and 30 mg/kg dose groups decreased, and the incidence of hepatic lateral nodules and septal hernia increased in the 30 mg/kg dose group (no examination was carried out in other dose groups): the vaginal opening of the offspring in the 10 and 30 mg/kg dose groups was delayed, and the reproductive function of the offspring in the 30 mg/kg dose group was impaired (fertility, luteal number, implantation and viable number were reduced, increased post-implantation loss, possibly due to effects on female offspring); maternal toxicity was seen in the 30 mg/kg dose group, but there was no evidence that the developmental effects of aripiprazole were secondary to maternal toxicity. Pregnant rats were given aripiprazole 3, 9 and 27 mg/kg/day intravenously during the organogenesis period. At high doses, fetal weight loss and delayed bone ossification were observed, and maternal toxicity occurred. Pregnant rabbits were orally given aripiprazole 10, 30 and 100 mg/kg/day (equivalent to 2, 3 and 11 times of MRHD in terms of AUC and 6, 19 and 65 times of MRHD in terms of mg/m) during the organogenesis period. Maternal food intake, abortion rate and fetal mortality rate decreased in the 100 mg/kg dose group, the 30 and 100 mg/kg dose groups reduced fetal body weight, increased incidence of skeletal abnormalities (sternal ganglion fusion), and intravenous aripiprazole 3, 10, and 30 mg/kg/day during the organogenesis of pregnancy. At the highest dose, significant maternal toxicity was produced, fetal body weight decreased, fetal abnormalities increased (mainly bone), and delayed fetal ossification increased, the non-affecting dose to the fetus is 10 mg/kg (equivalent to 5 times MRHD in terms of AUC and 6 times MRHD in terms of mg/m ")
Rats were given aripiprazole 3, 10 and 30 mg/kg/day (in mg/m, equivalent to 1, 3 and 10 times MRHD, respectively) orally during the perinatal period (from the 17th day of pregnancy to the 21st day after delivery). Mild maternal toxicity and mild prolongation of pregnancy were observed in the 30 mg/kg dose group, with increased stillbirth, reduced body weight (lasting to adulthood) and decreased survival rate. Rats were intravenously injected with aripiprazole 3, 8 and 20 mg/kg/day from the 6th day of pregnancy to the 20th day after delivery. The stillbirth rate increased in the 8 and 20 mg/kg/day dose groups, and the early fetal weight and survival rate decreased in the 8 and 20 mg/kg/day dose groups. These reactions occurred in the absence of maternal toxicity, and no effect on the postnatal behavior and reproductive function of the offspring was found.
Carcinogenicity
Lifetime carcinogenicity tests were performed on ICR mice, SD rats and F344 rats. The dosage of aripiprazole was 1, 3, 10 and 30 mg/kg/day for ICR mice, 1, 3 and 10 mg/kg/day for F344 rats, and 10, 20, 40 and 60 mg/kg/day for SD rats. In addition, SD rats were orally administered aripiprazole for 2 years at doses of 10, 20, 40, and 60 mg/kg/day (in mg/m, equivalent to 3 to 19 times MRHD). In male mice and rats, no tumorigenesis was observed. In female mice, the 3-30 mg/kg dose group (0.1-0.9 times MRHD in terms of AUC and 0.5-5 times MRHD in terms of mg/mR) had an increased incidence of pituitary adenomas, breast adenocarcinomas and adenoacanthomas. In female rats, the incidence of breast fibroadenoma increased at a dose of 10 mg/kg/day (0.1 times MRHD in terms of AUC and 3 times MRHD in terms of mg/m), and at a dose of 60 mg/kg/day (14 times MRHD in terms of AUC and 19 times MRHD in terms of mg/m "), the incidence of adrenal cortical carcinoma and adrenal cortical adenoma/carcinoma increased.
Hyperplastic changes in the pituitary and mammary glands of rodents were found after long-term administration of other antischizophrenic drugs and are thought to be prolactin-mediated. In the aripiprazole carcinogenicity test, serum prolactin levels were not measured. However, in the 13-week trial of repeated administration of the spiked diet, increased serum prolactin levels were observed in female mice at doses associated with breast and pituitary tumors. In the 4-week and 13-week trials of repeated administration of the spiked diet, there was no increase in serum prolactin levels in female rats at doses related to breast and pituitary tumors. The association of prolactin-mediated endocrine tumors in rodents with human risk is unclear.
young animal toxicity
Young rats were given aripiprazole 10, 20 and 40 mg/kg/day orally from weaning (21 days of age) to maturity (80 days of age). Aripiprazole caused death, central nervous system symptoms, impaired memory and learning ability, and delayed sexual maturity. Death, decreased mobility, hind limb expansion, arching, ataxia, tremor, and other central nervous system symptoms were observed in both male and female rats in the 40 mg/kg/day group. In addition, delayed sexual maturation was observed in male rats. Impaired memory and learning ability, increased spontaneous activity, and histopathological changes in the pituitary gland (atrophy), adrenal gland (hypertrophy of the adrenal cortex), mammary gland (hyperplasia and increased secretion), and female reproductive organs (vaginal mucous membrane liquefaction, atrophy of the endometrium, decreased number of corpus luteum of the ovary) were observed in all dose groups. Lesions of the female reproductive organs are thought to be secondary to elevated serum prolactin levels. No apparent adverse effect dose (NOAEL) could not be determined, and systemic exposure (AUC) to aripiprazole or its primary active metabolite at the lowest dose examined, 10 mg/kg/day, relative to the maximum recommend pediatric dose (15mg/day) in adolescents. There is no safety margin. After a 2-month recovery period, all drug-related effects were reversible, and most of the drug responses observed in young rats were also observed in large, previously conducted trials in adults,
When young dogs (2 months old) were given aripiprazole 3, 10 and 30 mg/kg/day by mouth for 6 months, the central nervous system symptoms such as tremor, decreased activity, ataxia, recumbency and limited hind limb activity were observed. Compared with the control group, the average body weight and body weight gain of the females in all the administration groups were reduced by up to 18%. The NOAEL could not be determined, and systemic exposure (AUC) to aripiprazole or its primary active metabolite at the lowest dose tested, 3 mg/kg/day, relative to the maximum recommend dose for children (15mg/day) in adolescents. There is no safety margin. After a 2-month recovery period, all drug-related effects were reversible.
Other Toxicity
Aripiprazole caused retinal degeneration in albino rats in a 26-week repeated toxicity test at a dose of 60 mg/kg and a 2-year carcinogenicity test at a dose of 40.60 mg/kg (13 and 19 times MRHD in mg/m? and 7 to 14 times MRHD in AUC). No retinal degeneration was induced in the white mice and monkeys. No other studies have been conducted to further evaluate its mechanism of action. The relevance of this result to human risk is unclear.
Drug abuse and dependence
Systematic studies of aripiprazole abuse, tolerance, or physical dependence have not been conducted in humans. In the monkey somatic dependence test, withdrawal symptoms were observed after abrupt drug withdrawal. The tendency to drug craving behavior has not been seen in clinical trials, but these studies are not systematic and it is not possible to predict, on the basis of this limited experience, the misuse, diversion and/or abuse of a CNS active drug once it is marketed. Therefore, patients should be carefully evaluated for drug abuse history and should be closely observed for signs of misuse or abuse (eg, drug resistance, increased drug use, drug craving behavior).
pharmacokinetics]
The activity of aripiprazole is mainly derived from the parent drug, aripiprazole, and to a lesser extent from its main metabolite, dehydroaripiprazole, which shows a similar affinity to the parent drug for the D, receptor, and the plasma content is 40% of the parent drug exposure. The mean elimination half-lives of aripiprazole and dehydroaripiprazole are approximately 75 and 94 hours, respectively. Steady state concentrations of both active ingredients were reached within 14 days of administration. The accumulation of aripiprazole can be predicted from its single-dose pharmacokinetics. At steady state, the pharmacokinetics of aripiprazole are proportional to the dose. Aripiprazole is mainly eliminated by liver metabolism, and the two P450 enzymes involved in metabolism are CYP2D6 and CYP3A4. For CYP2D6 low metabolizers, the average elimination half-way period of aripiprazole is about 146 hours.
Absorption
Oral solution: Aripiprazole oral solution is well absorbed. At the same dose, the plasma concentration of aripiprazole oral solution was higher than that of tablets. In a relative bioavailability study comparing the pharmacokinetic properties of 30mg aripiprazole oral solution with 30mg aripiprazole tablets in healthy subjects, the C and AUC of the oral solution were 122 and 114 of the tablets, respectively. The single-dose pharmacokinetics of aripiprazole is linear, and the performance is proportional to the dose in the dose range of 5-30mg.
Distribution
After intravenous administration, the steady-state volume of distribution of aripiprazole is high (404L or 4.9L/kg), indicating widespread distribution in the body. At therapeutic concentrations, more than 99% of aripiprazole and its main metabolites are bound to serum proteins, mainly serum albumin. Healthy male volunteers received aripiprazole 0.5-30mg/day for 14 consecutive days. Dose-dependent D-receptor binding indicates that aripiprazole can cross the blood-brain barrier.
metabolism and elimination
Aripiprazole is primarily metabolized by three biotransformation pathways: dehydrogenation, hydroxylation, and N-dealkylation. According to the results of in vitro experiments, CYP3A4 and CYP2D6 are involved in dehydrogenation and hydroxylation, and CYP3A4 is involved in N-dealkylation. Aripiprazole is the main drug component in the systemic circulation. At steady state, its active metabolite dehydroaripiprazole accounts for about 40% of aripiprazole AUC in plasma. About 8% of Caucasians and 3% to 8% of Africans/African business Americans lack the ability to metabolize CYP2D6 substrates and are classified as low metabolizers (PM) and others as adequate metabolizers (EM). Compared with EM, aripiprazole exposure of PM increased by about 80%, and the exposure of active metabolites decreased by about 30%. This results in an approximately 60% higher exposure of total active pharmaceutical ingredients of aripiprazole for PM than EM. Combined use of aripiprazole and CYP2D6 inhibitors, such as quinidine, in EM can lead to a 112% increase in aripiprazole plasma exposure, thus requiring dose adjustment. Similarly, PM has a higher plasma exposure to aripiprazole compared with EM, so PM should reduce its starting dose to 1/2. The average elimination half-life of aripiprazole in EM and PM is about 75 hours and 146 hours, respectively. Ali
Prazol does not inhibit or induce the CYP2D6 metabolic pathway, and approximately 25% and 55% of the radioactivity is recovered in urine and stools, respectively, after a single oral dose of ["C]-labeled aripiprazole. Less than 1% of the original drug is excreted through urine and 18% of the original drug is excreted through feces.
special population
Low liver function
In a single-dose trial (aripiprazole 15mg) in patients with different degrees of cirrhosis (Child-Pugh classification A, B, C), compared with healthy subjects, the AUC of aripiprazole was increased by 31% in subjects with mild hepatic impairment (HI), increased by 8% in subjects with moderate HI, and decreased by 20% in subjects with severe HI. None of these changes require dose adjustment.
In patients with severe renal impairment (creatinine clearance <30 mL/min), C increased by 36% and 53% for aripiprazole (single dose 15mg) and dehydroaripiprazole, respectively, but AUC decreased by 15% for aripiprazole and increased by 7% for dehydroaripiprazole. The renal excretion of aripiprazole original drug and dehydroaripiprazole is less than 1% of the dose. For subjects with low renal function, no dose adjustment is required.
The C and AUC of aripiprazole and its active metabolite-dehydroaripiprazole in female subjects were 30% to 40% higher than those in male subjects, and the apparent oral and femoral clearance of aripiprazole in female subjects was relatively lower. However, these differences can be largely explained by the difference in weight between men and women (25%), and dose adjustment for gender differences is not recommend. Race Although no specific pharmacokinetic studies of race factors have been performed, population pharmacokinetic evaluations of aripiprazole have not revealed clinically meaningful racial differences. There is no need to adjust the dose for racial differences.
Smoking According to the results of in vitro tests with human liver enzymes, aripiprazole is not a substrate for CYP1A2 and is not involved in direct glucuronidation. Therefore, smoking does not affect the pharmacokinetics of aripiprazole. Consistent with these in vitro results, the population pharmacokinetic evaluation did not show significant pharmacokinetic differences between smokers and non-smokers. There is no need to adjust the dose due to smoking status.
Shaded, sealed, stored not more than 25 "C,
[packaging]]
Oral liquid medicinal polyester bottle, 1 bottle/box.
Comes with polyethylene oral applicator.
[validity period]]
18 months. After the first opening, the maximum use of 3 months, and can not exceed the effective [implementation standard] of this product]
National Drug Administration Drug Registration Standards YBH16142023 [Approval No.] National Drug H20234454 [Marketing Authorization Holder]]
Company: Yueyang Xinhuada Pharmaceutical Co., Ltd. Registered Address: Wujiangqiao, Yueyang Economic and Technological Development Zone Postal Code: 414000
Telephone number: 0730-8258800
Fax number: 0730-8257700
Website: http://www.yyxhd.com [production enterprise]]
Company: Yueyang Xinhuada Pharmaceutical Co., Ltd. Production Address: Wujiangqiao, Yueyang Economic And Technological Development Zone Postal Code: 414000
Telephone number: 0730-8258800
Fax number: 0730-8257700
Website: http://www.yyxhd.com
Related Product recommend
online message
Filling in your phone and email information will help us get in touch with you in a timely manner and solve your problem as soon as possible.
 Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province
Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province  +86 730 8258800
+86 730 8258800  yyxhd_yt@vip.163.com
yyxhd_yt@vip.163.com

