
Bambuterol Hydrochloride Oral Solution
Key words:
Tablets, capsules, granules, topical solutions, oral solutions, suppositories, ointments
Classification:
Product Description
Bambuterol Hydrochloride Oral Solution Instructions
Please read the instructions carefully and use this product under the guidance of a physician. It is strictly prohibited to use it in food and feed processing.
[drug name] generic name: bambuterol hydrochloride oral solution English name: BambuterolHydrochloride 0ral
Solution
Hanyu Pinyin: Yansuan Banbuteluo KoufuRongye [ingredients]]
The main component of this product is bambuterol hydrochloride, chemical name: 1-[bis-(3 ',5'-N,N-dimethyl carbamoyloxy) phenyl]-2-N-tert-butyl aminoethanol hydrochloride. Excipients are sodium stevioside benzoate, citric acid, sodium citrate and orange flavor. Its structural formula is:
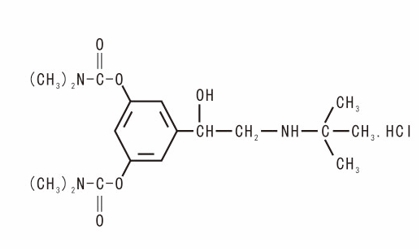 Molecular formula: C18H29N3O5· HCI
Molecular formula: C18H29N3O5· HCI
Molecular weight: 403.90
[character] this product is colorless to yellowish liquid, sweet taste
【Indications】 Bronchial asthma, chronic asthmatic bronchitis, obstructive emphysema and other lung diseases with bronchospasm.
"Specifications" 10ml:10mg
[usage and dosage] oral. Oral once every night before going to bed, the dose should be individualized. Over 12 years of age and adults: The starting dose of recommend is 10mg(1), which can be increased to 20mg(2) after 1 to 2 weeks of medication according to the clinical effect.
Children 6 to 12 years of age: the recommend dose is 10 mg(1 dose), not more than 10 mg(1 dose).
Children 2 to 5 years of age: the recommend dose is 5 mg (half), not more than 5 mg (half) is recommended.
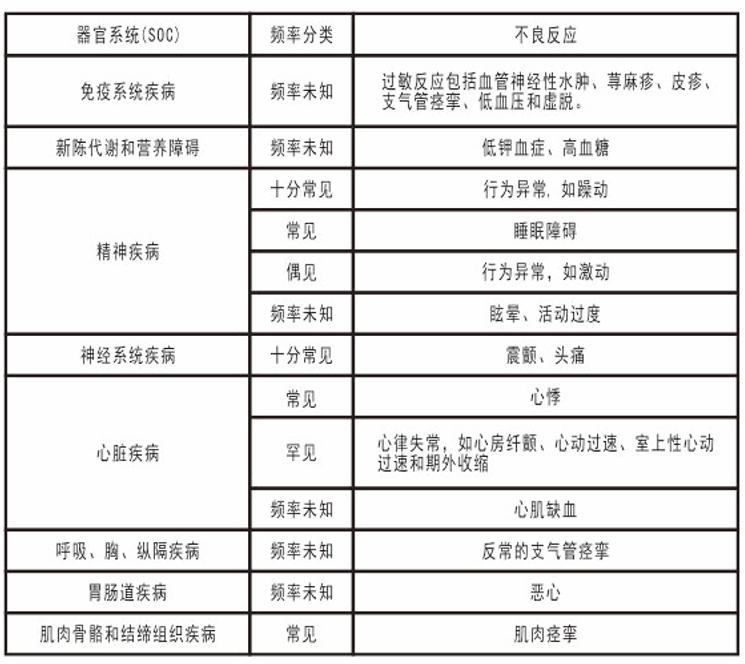
In patients with renal insufficiency (creatinine clearance <50 ml/min), the starting dose of recommend is 5mg (half a branch), which can be increased to 10mg(1 branch) after 1 to 2 weeks of medication according to the clinical effect. [Adverse reactions] Most adverse reactions belong to the characteristics of sympathomimetic amines, and the intensity of adverse reactions is dose-dependent. Tolerability of these adverse reactions usually develops after 1-2 weeks of treatment. Adverse reactions are shown by organ system and frequency. Frequency of occurrence is defined as: very common (>1/10), common (<1/10 and> 1/100), infrequent (<1/100 and> 1/1.000), rare (<1/1.000 and> 1/10,000), very rare (<1/10,000), and frequency unknown (not calculated from available data).
taboo]
Prohibited for allergy to this product, terbutaline, sympathomimetic amines and other ingredients in the formula. [Note]]
1. Since terbutaline is excreted mainly through the kidneys, the initial dose should be halved in patients with renal insufficiency (glomerular filtration rate ≤ 50 ml/min). In patients with cirrhosis or other severe hepatic impairment, the daily dose must be individualized given that the ability of the liver to metabolize benbuterol to terbutaline may be impaired. From a clinical point of view, direct use of the active metabolite terbutaline may be more appropriate in these patients. 3. As with all beta, receptor agonists, when used in patients with hyperthyroidism, need to be observed. Cardiovascular effects may occur during the use of sympathomimetic drugs (including this product). Based on some post-marketing data and published literature, there is evidence of a rare occurrence of myocardial ischemic events associated with B receptor agonists. Patients with potentially serious heart disease (e. g., ischemic heart disease, arrhythmias, or severe heart failure) should seek medical attention if they experience chest pain or other symptoms of worsening heart disease. Special attention should be paid to evaluating symptoms such as dyspnea and chest pain, as these may originate from the respiratory system or the heart. 5. Although the indication for this product does not include the treatment of preterm labor, it should be noted that benbuterol is metabolized to terbutaline, and terbutaline should not be used as a fetal protective drug for patients with ischemic heart disease or significant risk factors for ischemic heart disease. Due to the effect of β, receptor agonists on hyperglycemia, asthma patients with diabetes should strengthen blood glucose control when using this drug. 7. Because B, receptor agonists have a positive inotropic effect, patients with hypertrophic cardiomyopathy cannot use this drug.
8.B, Receptor agonists have the potential to be proarrhythmic and therefore the treatment of individual patients must be considered.
9.B, Treatment with receptor agonists may result in potentially severe hypokalemia. Patients with acute severe asthma attacks require special attention because the associated risk may be exacerbated by lack of oxygen. Concomitant treatment may also cause hypokalemic effects. (see [Drug Interactions]), so in this case, monitoring of serum potassium levels is recommended. Patients with persistent asthma who require maintenance therapy with B receptor agonists should also receive optimal anti-inflammatory therapy, such as inhaled corticosteroids and leukotriene receptor antagonists. Patients are advised to continue anti-inflammatory therapy even if their asthma symptoms are reduced after the start of treatment. If a previously effective dosing regimen does not relieve symptoms, this indicates worsening of the disease. Patients should seek further treatment and reassess treatment options. Additional treatment requirements (including increasing the dose of anti-inflammatory drugs) should be considered. Do not start treatment or increase your dose during an acute asthma attack. 11. Yi Hui angle-closure glaucoma patients should be treated with caution. 12. This product has no or negligible effect on the ability to drive and use machines.
[pregnant women and lactating women medication] no teratogenic effect of animal experiments, but it is recommended in the first three months of pregnancy with caution.
Due to the oral sustained-release beta, receptor agonists have anti-parturition effect in late pregnancy oral sustained-release B, receptor agonists in the treatment of asthma and other lung diseases should be used with caution.
It has been reported that mothers who use B, receptor agonists have transient hypoglycemia in premature infants and newborns. It is not known whether bamobuterol or its intermediate metabolites are secreted into milk, and terbutaline is secreted with milk, but does not adversely affect the infant at therapeutic doses. Decide whether it is necessary to stop breastfeeding or stop treatment with this product after weighing the benefits to the baby and the mother during treatment. [Children's medication] The dose for children under 2 years of age has not been determined, and [elderly medication] is the same as that for adults.
drug interaction]
1. With other sympathomimetic amine drug combination to strengthen the role of increased toxicity. 2 should not be combined with beta adrenergic receptor blockers (such as propranolol).
3. The muscle relaxant chlorosuccinylcholine (succinylcholine) is inactivated by plasma cholinesterase, and bambuterol can partially inhibit plasma cholinesterase, thereby prolonging the muscle relaxant effect of chlorosuccinylcholine (succinylcholine). This inhibition is dose-dependent, and the effect can be completely reversed after stopping bambuterol. This interaction also occurs with other muscle relaxants metabolized by cholinesterase. 4. Potassium reduction drugs and hypokalemia: Because B receptor agonists can cause hypokalemia, after careful evaluation of hypokalemia can increase the risk of arrhythmia, this product should be cautious when combined with serum potassium reduction drugs (such as diuretics, methylxanthines and glucocorticoids) that can increase the risk of hypokalemia. Hypokalemia is prone to digoxin toxicity.
B receptor blockers (including eye drops), especially those that are non-selective and may partially or completely inhibit B receptor agonists. Since quinidine can also inhibit cholinesterase in therapeutic doses in time, quinidine can theoretically inhibit the degradation of benbuterol. Six cases of angle-closure glaucoma caused by the combination of salbutamol and ipratropium bromide for the treatment of asthma (nebulized) have been reported. Similar to salbutamol, terbutaline may interact with ipratropium bromide when nebulized. Such co-administration is avoided in patients susceptible to angle-closure glaucoma. Halothane anesthetics should be avoided during treatment with B, receptor agonists because of the increased risk of arrhythmias. Caution should be exercised when other halogenated anesthetics are used with B, receptor agonists.
drug overdose]
Symptoms An overdose of this product may lead to an increase in the blood concentration of terbutaline, so its symptoms and signs are similar to those of an overdose of terbutaline, with the following manifestations: headache, anxiety, tremor, tonic muscle spasm, palpitations, tachycardia, and arrhythmias. When terbutaline is overdosed, a drop in blood pressure sometimes occurs. Laboratory reports: sometimes hyperglycemia and lactic acidosis.
High doses of beta, receptor agonists may cause hypokalemia by redistributing potassium in the body. An overdose of bainbuterol can also cause inhibition of plasma cholinesterase, which may last for several days.
Treatment of drug overdose
Treatment is usually not required. In severe overdose, the following measures may be considered depending on the case: gastric lavage and activated charcoal. Acid-base balance, blood glucose and electrolytes were measured. Monitor heart rate, heart rhythm and blood pressure. The most appropriate antidote to haemodynamically serious arrhythmias is a cardioselective B- blocker, but B- blockers must be used with caution in patients with a history of bronchospasm. If blood pressure drops due to a B, receptor-mediated decrease in peripheral vascular resistance, a blood volume-expanding agent must be administered.
pharmacology and toxicology]
Pharmacological action
This product is a prodrug of adrenaline B, a receptor agonist-terbutaline, which is metabolized to terbutaline under the action of enzymes after oral absorption. Terbutaline primarily activates B′ receptors, resulting in relaxation of bronchial smooth muscle. In addition, this product also has the effect of inhibiting the release of inflammatory mediators from mast cells.
Toxicity studies
Repeated toxicity: Wistar rats and Beagle dogs were given bambuterol hydrochloride at a dose of 108 mg/kg/d and 34.42 mg/kg/d, respectively, for 90 days. No toxicity was found in animal behavior, diet, hematology, blood biochemistry, gross anatomy, and histopathological examination of major organs. Genotoxicity: The results of Ames test, CHL cell chromosome aberration test and NIH mouse bone marrow micronucleus test were negative. Reproductive toxicity: bambuterol hydrochloride dose of 180 mg/kg/d, continuous oral administration for 10 days, no Wistar pregnant rats have obvious toxic reaction, no fetal appearance and visceral, bone deformity.
pharmacokinetics]
After oral administration of bambuterol hydrochloride, approximately 20% of the oral dose is absorbed. Simultaneous intake of food does not affect its absorption. It is slowly metabolized into active terbutaline after absorption. Bambuterol hydrochloride and intermediate metabolites show affinity for lung tissue, and metabolism of bambuterol hydrochloride → terbutaline also proceeds in lung tissue. Higher concentrations of active drug can thus be achieved in the lungs. After oral administration of the drug, the maximum blood concentration of the active metabolite terbutaline can be reached in about 7 hours, with a half-life of about 17 hours. In adults, 10% of the absorbed amount is converted to terbutaline. The clearance rate of terbutaline in children is lower than that in adults, but at the same time the amount of babuterol converted to terbutaline is also lower. Bambuterol hydrochloride and its metabolites, mainly excreted by the kidneys.
[storage] shading, airtight preservation.
[Packaging] Sodium calcium glass tube oral femoral liquid bottle, silicone rubber stopper for oral preparation, easy-to-stab aluminum cap for oral liquid bottle, 2 PCs/box, 4 PCs/box, 6 PCs/box.
[Validity Period] 24 Months [Implementation Standard] State Drug Administration Standard YBH01012019
[Approval No.] Chinese Medicine H20193122
[Listing Permit Holder] Yueyang Xinhuada Pharmaceutical Co., Ltd. [Address] Wujiang Bridge, Yueyang Economic and Technological Development Zone
[manufacturer] enterprise name: Yueyang Xinhuada Pharmaceutical Co., Ltd. Production address: Wujiang Bridge, Yueyang Economic and Technological Development Zone Postal Code: 414000
Telephone number: 0730-8258800
Fax number: 0730-8257700
Website: www.yyxhd.com
Related Product recommend
online message
Filling in your phone and email information will help us get in touch with you in a timely manner and solve your problem as soon as possible.
 Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province
Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province  +86 730 8258800
+86 730 8258800  yyxhd_yt@vip.163.com
yyxhd_yt@vip.163.com

