
Bisoprolol Fumarate Tablets
Key words:
Tablets, capsules, granules, topical solutions, oral solutions, suppositories, ointments
Classification:
Product Description
Bisoprolol Fumarate Tablets Instructions
Please read the instructions carefully and use them under the guidance of a physician.
[Name of Drug]]
Common name: Bisoprolol Fumarate Tablets English name: BisoprololFumarate Tablets Chinese Pinyin: Fumasuan Bisuoluo'er Pian
[ingredients]]
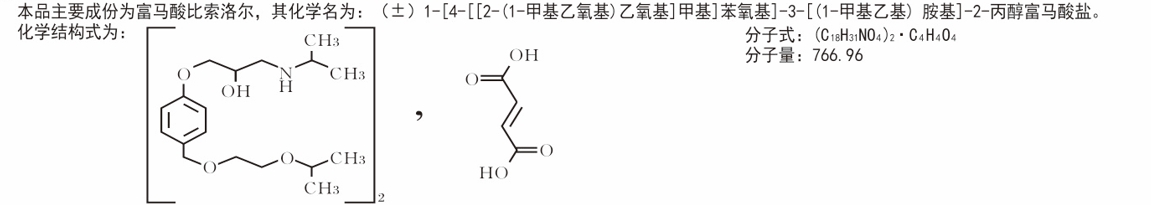
character]
This product is a light yellow shaped biconvex film-coated tablet with nicks in the center of both sides, which should be white after removing the coating.
indications]
Hypertension, coronary heart disease (angina). Chronic stable heart failure with reduced left ventricular systolic function (ejection fraction <35%). The use of this product requires medical treatment with ACE inhibitors, diuretics and selective use of cardiac glycosides.
[specification]]
5mg
[packing specification]]
12 tablets/24
operation channel]
OTC
[number of boxes/pieces]]
400
Retail Price/Box]
36/72
Product advantages and indications]
Patented new drug, the third generation of trace element supplements, regulate the dynamic balance of human body (trace) elements, form a closed loop that promotes each other, and achieve the purpose of tonic. Is the Chinese family standing medicine on the list of brand indications: for zinc, iron, calcium deficiency caused by a variety of related diseases.
5mg
【usage dosage]
For all indications: This product should be taken in the morning and can be taken with meals. Take the whole piece of water and should not be chewed. This product should be used according to the doctor's prescription.
Treatment of hypertension or angina pectoris: usually 5mg bisoprolol fumarate once a day. Patients with mild hypertension can start treatment from 2.5mg bisoprolol fumarate. If the effect is not obvious, the dose can be increased to 10mg bisoprolol fumarate once a day. The dosage of this product should be adjusted according to individual conditions, and special attention should be paid to the pulse and therapeutic effect. This product should be long-term medication. The dose of this drug cannot be changed without medical advice, and it is not advisable to stop taking the drug. If you need to stop the drug. Should be gradually stopped, not suddenly interrupted. Patients with coronary heart disease need special attention. Treatment of chronic stable heart failure (HF): The patient must be stable (no acute failure) at the time of initiation of bisoprolol therapy. Standard treatment for chronic heart failure includes an ACE inhibitor (or an angiotensin receptor blocker if an ACE inhibitor is intolerant), a beta-blocker, a diuretic, and, as appropriate, a cardiotonic. The treatment of chronic stable heart failure requires a special dose titration period. It is recommended to use this product under the guidance of a doctor with experience in the treatment of chronic heart failure.
Note: When treating chronic stable heart failure, there must be a dose titration phase, starting with a low dose and gradually increasing according to the following scheme:
◆ 1.25mg once daily for 1 week, if well tolerated, increase
◆ 2.5mg once daily for 1 week, if well tolerated, increase
◆ 3.75mg once daily for 1 week, if well tolerated, increase
◆ 5mg once daily for 4 weeks, if well tolerated, increase
◆ 7.5mg once daily for 4 weeks, if well tolerated, increase
◆ 10mg, once a day, the maximum recommend dose for maintenance treatment is 10mg, once a day. Close monitoring of vital signs (blood pressure, heart rate), conduction block, and symptoms of worsening heart failure after the first dose and during dose escalation is recommended. Dose Adjustment If there is a temporary worsening of heart failure, hypotension, or bradycardia, it is recommended that the concomitant dose be reconsidered. If necessary, the dose of bisoprolol may be temporarily reduced, or discontinuation may be considered. Restarting bisoprolol therapy and/or increasing the dose of bisoprolol was considered when the disease was stable. The use of this product in the treatment of chronic stable heart failure should be long-term medication. Do not stop or change the dose abruptly without the guidance of a physician. Because it may cause a temporary worsening of the condition. Especially for patients with ischemic heart disease should not suddenly stop the drug. If you need to stop the drug, it is recommended to gradually reduce the dose. special population
Hepatic and renal insufficiency
Treatment of hypertension or angina pectoris: Patients with mild to moderate hepatic and renal insufficiency usually do not require dose adjustment. Patients with severe renal failure (creatinine clearance <20 ml/min) and severe liver dysfunction.
The daily dose should not exceed 10mg.
There is less experience with the use of bisoprolol in patients on renal dialysis; however, there is no evidence that the dose needs to be adjusted in this group of patients.
Treatment of chronic stable heart failure: There are no pharmacokinetic data for bisoprolol in patients with chronic heart failure and associated hepatic and renal dysfunction. Dose escalation in such patients should be particularly cautious.
Elderly patients: Dose adjustment is not required.
[Adverse Reactions] The following adverse reactions are classified according to system organs, and the incidence is defined as follows:
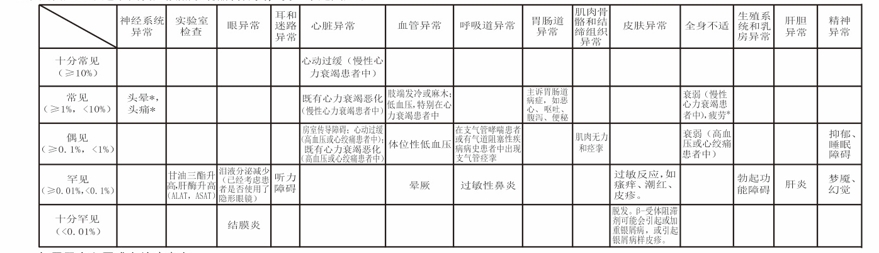
Only for patients with hypertension or angina pectoris: * These symptoms occur especially at the beginning of treatment. It is usually mild and usually disappears after 1-2 weeks. Inform your physician of any of the above adverse reactions or any unexpected reactions. To avoid serious reactions, inform your physician immediately when an adverse reaction is severe, sudden, or rapidly worsening.
Bisoprolol is contraindicated in the following patients: 1. Patients with acute heart failure or decompensated heart failure requiring intravenous inotropic drugs 2. Patients with cardiogenic shock 3. Patients with second or third degree atrioventricular block (without cardiac pacemaker) 4. Patients with sick sinus syndrome 5. Patients with sinoatrial block 6. Patients with symptomatic bradycardia (symptomatic bradycardia) 7. Patients with symptomatic hypotension 8. Severe bronchial asthma 9. Severe peripheral arterial occlusive disease and. Patients with untreated pheochromocytoma 11. Patients with metabolic acidosis 12. Patients with known hypersensitivity to bisochromocytoma and its derivatives or any of its components
[Precautions] Treatment of chronic stable heart failure with bisoprolol must start with a specific dose titration period and should be monitored regularly. Because it may cause short-term deterioration of heart disease, unless there is clear evidence, especially for patients with ischemic heart disease should not suddenly stop the drug. Bisoprolol should be used with caution in patients with hypertension or angina accompanied by heart failure. Special attention should be paid when using this product in the following situations:
◆ Large fluctuations in blood sugar levels in patients with diabetes; may mask symptoms of hypoglycemia
◆ Strict fasting
◆ Desensitization treatment in progress
◆ Variant angina ◆ Peripheral arterial occlusive disease (symptoms may worsen, especially at the beginning of treatment) First degree atrioventricular block Although cardiac selective (β,)β-blockers may have less effect on lung function than non-selective β-blockers, like all β-blockers, they should be avoided in patients with airway obstructive diseases unless necessary. If used in such patients, special care should be taken when using this product. In patients with airway obstructive cold disease, bisoprolol should be administered from the lowest possible dose and closely monitored for symptoms (eg, dyspnea, decreased activity tolerance, cough) so that bronchiectasis should be administered at the same time. Use of this product in asthmatic patients Occasional use of this product in patients with tracheal asthma and other chronic obstructive pulmonary diseases may cause the corresponding symptoms and increased airway resistance, so the dose of βz-agonist should be increased. Like other beta-blockers, bisoprolol may increase the body's sensitivity to allergens and aggravate allergic reactions. Adrenaline treatment at this time does not necessarily produce the desired therapeutic effect. General anesthesia: When the patient is under general anesthesia, the anesthesiologist must be informed that the patient is using a beta-blocker. If it is considered necessary to stop the drug before surgery, the drug should be gradually stopped and anesthesia should be carried out after 48 hours of complete withdrawal. Patients with psoriasis or a family history of silver eyebrow disease can only decide whether to use beta-blockers (such as bisoprolol fumarate tablets) after careful consideration of the pros/cons. Patients with pheochromocytoma can be treated with bisoprolol only after the use of a-blockers.
Treatment with bisoprolol may mask the symptoms of thyrotoxicosis. In a study of patients with coronary heart disease, bisoprolol did not affect the patient's ability to drive. However, due to individual differences in response to efficacy, the use of this product may affect the ability to drive or manipulate machinery. Especially in the beginning of medication, increase the dose and with alcohol should be more attention. There is no experience with bisoprolol in the treatment of heart failure with the following diseases or conditions: ◆ Insulin-dependent diabetes mellitus (type I) ◆ Severe renal impairment ◆ Severe hepatic impairment ◆ Congenital heart disease ◆ Athletes with organic valvular disease with significant hemodynamic changes Use with caution. Pregnant women and lactating women: Bisoprolol may damage pregnant women and/or fetus/newborn. In general, beta-adrenoceptor antagonists reduce placental perfusion, which is associated with growth retardation, intrauterine death, miscarriage, and preterm birth; adverse effects such as hypoglycemia and bradycardia may occur in the fetus and newborn. If a β-adrenoceptor blocker must be used, a selective B- adrenoceptor blocker is desirable. Bisoprolol should not be used in pregnant women unless it is clear that it must be used. If treatment with bisoprolol is necessary, uteroplacental blood flow and fetal growth should be monitored. Once a harmful effect on the pregnant woman and fetus is found, other treatment methods should be selected. Neonates must be closely monitored, and symptoms such as hypoglycemia and bradycardia are most likely to occur in the first 3 days of life. Lactating women: It is not clear whether this product is excreted by human milk. Therefore, bisoprolol is not recommended for lactating women. [Children's medication] There is no experience in the application of bisoprolol in pediatric patients, so this product is not recommended for children. There is no need to adjust the dose,
Restrictive cardiomyopathy ◆ Myocardial infarction within 3 months
[Drug interaction], non-recommend concomitant medication: used for the treatment of chronic stable heart failure Class 1 antiarrhythmic drugs (such as disopyramide, quinidine): may increase the inhibitory effect of this product on atrioventricular conduction and cardiac contractility. Calcium antagonists such as verapamil and diltiazem for all indications: negative effects on contractility, atrioventricular conduction and blood pressure. Intravenous administration of verapamil in patients treated with beta-blockers can cause significant hypotension and atrioventricular block. Central antihypertensive drugs (eg, clonidine, methyldopa, moxonidine, limenidine) may cause heart rate due to decreased central sympathetic tone
and decreased cardiac output and vasodilation. Abrupt withdrawal, especially before stopping beta-blockers, may increase the risk of "rebound hypertension. 2. Concomitant drugs to be used with caution for the treatment of hypertension or angina pectoris: Class 1 antiarrhythmic drugs (such as disopyramide, quinidine): may increase the inhibitory effect of this product on atrioventricular conduction and cardiac contractility. Calcium antagonists such as dihydropyridine derivatives (eg, nifedipine): Concomitant use increases the risk of hypotension and further deterioration of ventricular pump function in patients with heart failure who cannot be ruled out. Class III Antiarrhythmic drugs (eg, amiodarone): May prolong atrioventricular conduction time. Parasympathomimetic drugs (including tetrahydroaminoacridine): May prolong atrioventricular conduction time and increase the risk of bradycardia. Other B- blockers, including eye drops, may enhance their effects. Insulin and oral antidiabetic drugs: increased hypoglycemic effect. Blocking B- adrenoceptors may mask hypoglycemic symptoms. Anesthetics: May cause a decrease in reflex tachycardia, increasing the risk of hypotension. Digitalis II: Slow heart rate, prolong atrioventricular conduction time, non-steroidal anti-inflammatory drugs (NSAIDs) may weaken the hypotensive effect of this product. B- Sympathomimetic drugs (eg, isoproterenol, dobutamine): When combined with bisoprolol, the effect of these two drugs may be reduced. Co-administration of sympathomimetic drugs (eg, norepinephrine, epinephrine) that activate both β-and α-adrenoceptors may exacerbate the α-adrenoceptor-mediated vascular incorporation of these drugs, resulting in increased blood pressure and increased intermittent claudication. It is generally believed that such interactions are more likely to occur when non-selective B- receptor positives are used. When combined with antihypertensive drugs and other drugs that may lower blood pressure (eg, tricyclic antidepressants, barbiturates, phenothiazines), it may increase the risk of hypotension.
3. Concomitant medication mefloquine: may increase the risk of bradycardia. Monoamine oxidase inhibitors (except MA0-B inhibitors): may increase the hypotensive effect of β-blockers and may also increase the risk of hypertensive crisis.
drug overdose]
The most common overdose reactions to B- adrenoceptor blockers are bradycardia, hypotension, bronchial asthma, acute cardiac insufficiency, and hypoglycemia. Individual variation in sensitivity to a single high dose of bisoprolol is large, and patients with heart failure may be very sensitive. Drug overdose usually occurs and should be promptly discontinued and supportive symptomatic treatment given. Limited data indicate that bisoprolol is difficult to remove by dialysis. Based on the expected pharmacokinetics and experience with other beta-blockers, the following management may be considered when clinically necessary: Bradycardia: Instilled atropine. If the effect is not good, isoproterenol or other positive chronotropic drugs can be given with care. In some cases, a pacemaker should be implanted through a vein. Hypotension: intravenous infusion of fluid and vasopressor drugs, intravenous injection of glucagon is beneficial. Atrioventricular block (second or third degree): the patient should be carefully monitored, appropriate intravenous infusion of isoproterenol or implantation of cardiac pacemakers through the vein. Acute heart failure exacerbation: intravenous diuretics, inotropic drugs and vasodilators. Bronchospasm: treatment with bronchodilators, such as isoproterenol,-sympathomimetics and/or aminophylline. Hypoglycemia: intravenous glucose. pharmacology and toxicology]
Pharmacological action
Bisoprolol is a highly selective β,-adrenoceptor blocker (cardioselective) with no intrinsic sympathomimetic and membrane stabilizing activity in the therapeutic dose range. Bisoprolol Fumarate in
Beyond therapeutic doses (>20mg), beta-adrenoceptors on bronchial and vascular smooth muscle are also inhibited. Therefore, in order to maintain high selectivity to the heart, it is important to use the lowest effective dose.
toxicological studies
Genotoxicity: Bisoprolol Ames test, Chinese hamster V79 cell gene mutation test and chromosome aberration test, DNA damage test, rat cell genetic test and mouse micronucleus test.
The fruits were all negative.
Reproductive toxicity: when bisoprolol fumarate is orally administered to rats at a dose of 150 mg/kg/d, there is no obvious effect on fertility and early embryo development, which is ten thousand times the maximum dose of human recommend (MRHD) (in the embryo-fetal developmental toxicity test calculated by body surface area, when bisoprolol fumarate is orally administered to pregnant rats at a dose of 50 mg/kg/d, the incidence of embryo absorption can be increased, the dose is 26 times that of MRHD. The maternal toxicity (reduction of food intake and inhibition of weight gain) dose is 150 mg/kg/day, which is 77 times that of MRHD. When bisoprolol fumarate is given orally during pregnancy up to 12.5 mg/kg/d, the early absorption rate of embryos can be seen to increase, and no fetal development toxicity is found. The dose is 12 times that of MRHD.
Carcinogenicity: mice were given oral bisoprolol fumarate at a dose of 250 mg/kg/d for 20 and 24 months, and rats were given oral bisoprolol fumarate at a dose of 125 mg/kg/d for 26 months, with no drug-related carcinogenicity. The above doses were calculated as body surface area and were 59 and 64 times the MRHD, respectively. Pharmacokinetics Bisoprolol is almost completely absorbed (>90%) in the gastrointestinal tract. Since the first-pass effect is small (<10%), it exhibits a bioavailability of up to about 90%. The plasma protein binding rate of bisoprolol is about 30%, the volume of distribution is 3,5 liters/kg, and the total clearance is about 15 liters/hour. The plasma half-life after once daily administration is 10-12 hours and can be maintained in plasma for 24 hours. Bisoprolol is excreted from the body through two routes. 50% is metabolized by the liver to inactive metabolites and then excreted from the kidneys, and the remaining 50 * is excreted from the kidneys in the form of a prototype drug. Because the proportion of drug clearance from the kidney and liver is the same, patients with mild to moderate liver and kidney dysfunction do not need to adjust the dose. There are no studies on pharmacokinetics in patients with chronic stable heart failure with impaired hepatic function or renal insufficiency.
The kinetics of bisoprolol was linear and independent of age. Patients with chronic heart failure (NYHA class I 1) have higher plasma bisoprolol levels and longer half-lives than healthy volunteers. The maximum plasma concentration at steady state after 10m/day was 64 21ng/ml, with a half-life of 17 5 hours. [Storage] sealed below 25C. [packaging] PVC solid pharmaceutical hard sheet/aluminum foil for pharmaceutical packaging, polyester/aluminum/polyethylene pharmaceutical composite film, bag. 12 pieces/plate x1 plate/bag/box; 12 pieces/plate x2 plate/bag/box; 15 pieces/plate/bag/box; 10 pieces/plate/bag/box; 9 pieces/plate x2 plate/bag/box; 15 pieces/plate x2 plate/bag/box.
[Validity] 24 months. Standard YBH07222020 of State Drug Administration
[Approval No.] Sinopharm Zhunzi H20173037 [Drug Marketing License Holder] Enterprise Name: Yueyang Xinhuada Pharmaceutical Co., Ltd. Production Address: Wujiang Bridge, Yueyang Economic and Technological Development Zone
Telephone number: 0730-8258800 Fax number: 0730-8257700 Website: http://waw.yyxhd.com
Enterprise Name: Yueyang Xinhuada Pharmaceutical Co., Ltd. Production Address: Wujiang Bridge Postal Code of Yueyang Economic and Technological Development Zone: 414000 Telephone Number: 0730-8258800 Fax Number: 0730-8257700 Website: http://www.yyxhd.com
Related Product recommend
online message
Filling in your phone and email information will help us get in touch with you in a timely manner and solve your problem as soon as possible.
 Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province
Wujiang Bridge, Yueyang Economic and Technological Development Zone, Hunan Province  +86 730 8258800
+86 730 8258800  yyxhd_yt@vip.163.com
yyxhd_yt@vip.163.com

